2nd Feb 2022
 Brain Insights is the dedicated student section published in the British Neuroscience Association (BNA) Bulletin. It represents the voice of the BNA student: written by students for students. In this student article, postgraduate Archie Leitch explores cannabis as a treatment for mental health conditions.
Brain Insights is the dedicated student section published in the British Neuroscience Association (BNA) Bulletin. It represents the voice of the BNA student: written by students for students. In this student article, postgraduate Archie Leitch explores cannabis as a treatment for mental health conditions.
Writing for Brain Insights is now a members-only benefit. If you're not already a member you can join here from just £1 a month.
 Cannabis is the most prevalent illegal substance used in the world today (1) and represents a drug class with use spanning diverse cultural, recreational and medicinal settings. Like many illicit compounds, its reputation is embodied by themes of criminality and poor mental health. However, understanding the endogenous and exogenous applications of cannabinoids (molecules primarily interacting with cannabinoid receptors) has the capacity to drastically aid a current mental health crisis - depression. At present, the utility of cannabinoids for treating conditions such as depression is in-optimal, as we will explore in the case of the recent approval and subsequent removal of the antiobesity drug, rimonabant.
Cannabis is the most prevalent illegal substance used in the world today (1) and represents a drug class with use spanning diverse cultural, recreational and medicinal settings. Like many illicit compounds, its reputation is embodied by themes of criminality and poor mental health. However, understanding the endogenous and exogenous applications of cannabinoids (molecules primarily interacting with cannabinoid receptors) has the capacity to drastically aid a current mental health crisis - depression. At present, the utility of cannabinoids for treating conditions such as depression is in-optimal, as we will explore in the case of the recent approval and subsequent removal of the antiobesity drug, rimonabant.
Rimonabant: the problematic transition into medicinal cannabinoids
The discovery of the endocannabinoid system and its two cannabinoid receptors brought promising novel drug targets, with initial pharmacological approaches attempting to attenuate the obesity-associated symptom of excessive appetite (2, 3). Marijuana is composed of CB receptor agonists which increase the palatability of unhealthy food. The French multinational healthcare provider Sanofi Aventis hoped to reverse this effect via by developing a CB1 inverse agonist, rimonabant. In 2006, the European Medicines Agency (EMA) approved rimonabant for obese and overweight individuals, following phase-3 data showing its efficacy in reducing caloric intake, see Figure 1a (4, 5).
However, by 2008 Rimonabant had been rejected by the Food and Drug Administration (FDA) and was withdrawn from the market by the EMA. Provisional results indicated a significant increase in depressive behaviours in patients, including suicidal ideation and severe low mood (6). This raised important questions surrounding our capacity to predictively pharmacologically modulate mental health – why does rimonabant give this effect? Could this effect have been predicted? Finally, could the relationship between cannabinoids and mental health represent an affective therapeutic opportunity?
Top
Preclinical affective characterisation of rimonabant
Our neurological understanding of depression is incomplete; however, it is agreed that pathology includes depressed monoaminergic transmission (7). Despite the dense expression of CB receptors in affective neuronal networks, extensive research does not suggest a depressive effect of CB inverse agonism (8), in fact, it suggests the opposite.
For example, in rat ex-vivo prefrontal cortical neurons, cannabinoid inverse agonism improves acetylcholinergic transmission, supporting prior research analysing noradrenaline and dopamine release in hippocampal and striatal slices (9, 10). Increases in monoaminergic activity have also translated to human cortical slices, where rimonabant induces similar effects as established antidepressants (11, 12). This surprising preclinical evidence is also represented by various in-vivo behavioural investigations (Figure 1b), where rimonabant acted as an antidepressant in both mice and gerbils via acute or chronic dosing, paired with similar neurotransmitter mechanisms as seen ex vivo (13, 11, 14). The role of CB antagonism in these studies was confirmed by genetic knockout assays, which removed these behavioural changes (15).
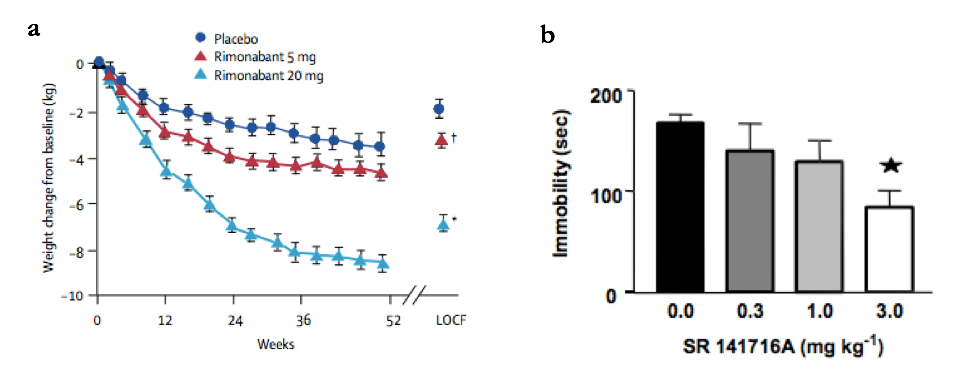
Figure 1: Rimonabant increases weight loss in obese patients and decreases immobility time in the forced swim test (FST)
(a) Rimonabant dosed once daily at 5mg (red) and 20mg (light blue) gave an increased weight loss in obese patients over 1 year, when compared to placebo (dark blue). Significant differences from placebo were measured when comparing LOCF (last observation carried forward) values, a statistical method which extrapolates final data points from previously measured trends. *p=0.001, †p=0.002 (vs placebo). Taken from (4).
(b) Increasing the I.P injected dose of rimonabant (SR141716A) reduced mouse immobility time (time without swimming) in the FST, with a significant difference at 3.0mg/kg compared to control. *p < 0.05 (11). Data represents mean +/- SEM. Taken from (11).
Top
The complexities surrounding cannabis and depression
Interestingly, excitation as well as inhibition of CB receptors predicts antidepressant effects in preclinical models. This has been shown via in-vivo analyses (forced swim and tail suspension
tests) and ex-vivo monoaminergic assays modelling attenuated endocannabinoid activity (16, 17). Conflicting findings also extend to human studies of this relationship. Of note, heightened prefrontal CB receptor expression was found in suicide victims (18), suggesting compensation following reduced endocannabinoid activity, which supports findings using serum biomarkers of CB activity. However, evidence also suggests a reduced receptor expression, as well as non-significant differences in CB receptor number, in clinically depressed brains compared to healthy samples (19, 20, 21). Additional conflicting evidence also suggests enhancing endocannabinoidergic activity improves mental health, following measurements showing electroconvulsive therapy for depression increases endocannabinoid activity (22). Studies collectively indicate both the positive and negative and influence of cannabis on depression, which is complicated by a lack of causal conclusions and the involvement of extensive contextual factors (23, 24, 25).
Top
Why is research inconclusive and what can be done about it?
This lack of confirmational research is embodied by a range of issues, most prominently; translation of preclinical research and the legality of cannabinoids. The ability of preclinical assays to represent human mental health is notoriously poor, where disparity spanning from in-vitro to behavioural research gives rise to serious health implications, as was seen with rimonabant. Common assays modelling depression (forced swim and tail suspension tests) are now discouraged due to limited translation (26), highlighting the prevalence of inapplicable research to our efforts in treating affective disorders. The necessity to rethink our approach is represented by cannabinoids and depression, which, if investigated correctly, holds the potential to improve the treatment of depression. Here, pioneering work includes assays with improved translation, such as the affective bias test and ambiguous cue interpretation (27).
The need for alternative mental health approaches is growing, and as such, a social and academic revolution surrounding previously abandoned compounds is spreading, supported by innovative trials showing the cross-indication efficacy of psychedelics (28). Similarly to cannabinoids, the legal and social implications impose a hinderance to research, with restrictions surrounding funding and social acceptance of illicit drug use (29). In addition to poor preclinical translation, this has contributed to our lack of success in harnessing the capabilities of cannabinoids.
The capacity for cannabinoids to alter mental health is apparent, and although the most informed and therapeutic approach toward using these molecules is yet to be found, this field represents an opportunity to benefit those most in need.
References
1. Marshall, K., Gowing, L., Ali, R. and Le Foll, B., 2014. Pharmacotherapies for cannabis dependence. Cochrane Database of Systematic Reviews, (12).
2. Pertwee RG. Cannabinoid Receptors. London, United Kingdom: Academic Press, 1995. 264 p. (Neuroscience Perspectives).
3. Foltin, r. W., Brady, J. V. & Fischman, M. W, 1986. Behavioral analysis of marijuana effects on food intake in humans. Pharmacol. Biochem. Behav. 25, 577–582.
4. Van Gaal, L.F., Rissanen, A.M., Scheen, A.J., Ziegler, O., Rössner, S. and RIO-Europe Study Group, 2005. Effects of the cannabinoid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1-year experience from the RIO-Europe study. The Lancet, 365(9468), pp.1389-1397.
5. Pi-Sunyer, F.X., Aronne, L.J., Heshmati, H.M., Devin, J., Rosenstock, J. and RIO-North America Study Group, F., 2006. Effect of rimonabant, a cannabinoid-1 receptor blocker, on weight and cardiometabolic risk factors in overweight or obese patients: RIO-North America: a randomized controlled trial. Jama, 295(7), pp.761-775.
6. Amir H. Sam, Victoria Salem, Mohammad A. Ghatei, 2011 "Rimonabant: From RIO to Ban", Journal of Obesity, vol. 2011, Article ID 432607, 4 pages.
7. Hindmarch, I., 2001. Expanding the horizons of depression: beyond the monoamine hypothesis. Human Psychopharmacology: Clinical and Experimental, 16(3), pp.203-218.
8. Howlett, A.C., Barth, F., Bonner, T.I., Cabral, G., Casellas, P., Devane, W.A., Felder, C.C., Herkenham, M., Mackie, K., Martin, B.R. and Mechoulam, R., 2002. International
Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacological reviews, 54(2), pp.161-202.
9. Kathmann, M., Bauer, U., Schlicker, E. and Göthert, M., 1999. Cannabinoid CB1 receptor-mediated inhibition of NMDA-and kainate-stimulated noradrenaline and dopamine release in the brain. Naunyn-Schmiedeberg's archives of pharmacology, 359(6), pp.466-470.
10. Gifford, A.N. and Ashby, C.R., Jr., 1996. Electrically evoked acetylcholine release from hippocampal slices is inhibited by the cannabinoid receptor agonist, WIN 55212-2, and is potentiated by the cannabinoid antagonist, SR 141716A. J. Pharmacol. Exp. Ther. 277, 1431–1436
11. Tzavara, E.T., Davis, R.J., Perry, K.W., Li, X., Salhoff, C., Bymaster, F.P., Witkin, J.M. and Nomikos, G.G., 2003. The CB1 receptor antagonist SR141716A selectively increases monoaminergic neurotransmission in the medial prefrontal cortex: implications for therapeutic actions. British journal of pharmacology, 138(4), pp.544-553.
12. Tanda, G. et al. (1994) Increase of extracellular dopamine in the prefrontal cortex: a trait of drugs with antidepressant potential? Psychopharmacology (Berl.) 115, 285–288
13. Shearman, L.P., Rosko, K.M., Fleischer, R., Wang, J., Xu, S., Tong, X.S. and Rocha, B.A., 2003. Antidepressant-like and anorectic effects of the cannabinoid CB1 receptor inverse agonist AM251 in mice. Behavioural pharmacology, 14(8), pp.573-582.
14. Griebel, G., Stemmelin, J. and Scatton, B., 2005. Effects of the cannabinoid CB1 receptor antagonist rimonabant in models of emotional reactivity in rodents. Biological psychiatry, 57(3), pp.261-267.
15. Witkin, J.M., Tzavara, E.T., Davis, R.J., Li, X. and Nomikos, G.G., 2005. A therapeutic role for cannabinoid CB1 receptor antagonists in major depressive disorders. Trends in pharmacological sciences, 26(12), pp.609-617.
16. Hill MN, Gorzalka BB, 2005. Pharmacological enhancement of cannabinoid CB1 receptor activity elicits an antidepressant-like response in the rat forced swim test. European Neuropsychopharmacology. 15: 593-599. 10.1016/j.euroneuro.2005.03.003
17. Gobbi G, Bambico FR, Mangieri R, Bortolato M, Campolongo P, Solinas M, Cassano T, Morgese MG, Debonnel G, Duranti A, Tontini A, Tarzia G, Mor M, Trezza V, Goldberg SR, Cuomo V, Piomelli D., 2005. Antidepressant-like activity and modulation of brain monoaminergic transmission by blockade of anandamide hydrolysis. PNAS. 102 (51): 18620-18625. 10.1073/pnas.0509591102.
18. Hungund, B.L., Vinod, K.Y., Kassir, S.A., Basavarajappa, B.S., Yalamanchili, R., Cooper, T.B., Mann, J.J. and Arango, V., 2004. Upregulation of CB 1 receptors and agonist-stimulated [35 S] GTP γ S binding in the prefrontal cortex of depressed suicide victims. Molecular psychiatry, 9(2), pp.184-190.
19. Hill MN, Miller GE, Ho WS, Gorzalka BB, Hillard CJ. Serum endocannabinoid content is altered in females with depressive disorders: a preliminary report. Pharmacopsychiatry.
2008 Mar;41(2):48-53. doi: 10.1055/s-2007-993211. PMID: 18311684; PMCID: PMC3422568.
20. Choi K, Le T, McGuire J, Xing G, Zhang L, Li H, Parker CC, Johnson LR, Ursano RJ. Expression pattern of the cannabinoid receptor genes in the frontal cortex of mood disorder patients and mice selectively bred for high and low fear. J Psychiatr Res. 2012 Jul;46(7):882-9.
21. García-Gutiérrez MS, Navarrete F, Navarro G, Reyes-Resina I, Franco R, Lanciego JL, Giner S, Manzanares J. Alterations in Gene and Protein Expression of Cannabinoid CB2 and GPR55 Receptors in the Dorsolateral Prefrontal Cortex of Suicide Victims. Neurotherapeutics. 2018 Jul;15(3):796-806. doi: 10.1007/s13311-018-0610-y. PMID: 29435814; PMCID: PMC6095782.
22. Kranaster, L., Hoyer, C., Aksay, S.S., Bumb, J.M., Leweke, F.M., Janke, C., Thiel, M., Lutz, B., Bindila, L. and Sartorius, A., 2017. Electroconvulsive therapy enhances endocannabinoids in the cerebrospinal fluid of patients with major depression: a preliminary prospective study. European archives of psychiatry and clinical neuroscience, 267(8), pp.781-786.
23. Walsh, Z., Gonzalez, R., Crosby, K., Thiessen, M.S., Carroll, C. and Bonn-Miller, M.O., 2017. Medical cannabis and mental health: A guided systematic review. Clinical psychology review, 51, pp.15-29.
24. Degenhardt, L., Hall, W. and Lynskey, M., 2003. Exploring the association between cannabis use and depression. Addiction, 98(11), pp.1493-1504.
25. Kosiba JD, Maisto SA, Ditre JW. Patient-reported use of medical cannabis for pain, anxiety, and depression symptoms: Systematic review and meta-analysis. Soc Sci Med. 2019 Jul;233:181-192. doi: 10.1016/j.socscimed.2019.06.005. Epub 2019 Jun 8. PMID: 31207470.
26. Reardon, S., 2019. Depression researchers rethink popular mouse swim tests. Nature, 571(7766), pp.456-458.
27. Robinson, E.S., 2016. Improving the translational validity of methods used to study depression in animals. Psychopathology Review, 3(1), pp.41-63.
28. Nutt, D. and Carhart-Harris, R., 2021. The current status of psychedelics in psychiatry. JAMA psychiatry, 78(2), pp.121-122.
29. Hill, K.P., 2019. Medical use of cannabis in 2019. Jama, 322(10), pp.974-975.
Top
Want to write for Brain Insights?
Brain Insights welcomes contributions from our undergraduate and postgraduate students, Career Starter and early ECR members who are interested in writing.
To express an interest in writing an article, please contact one of our editors below:
- Harriet Hobday: harriet.hobday@kcl.ac.uk
- Ariane Delgado Sanchez: ariane.delgadosanchez@manchester.ac.uk
- Ryan Stanyard: ryan.a.stanyard@kcl.ac.uk
Writing for Brain Insights is now a members' only benefit. If you're not already a member you can join here.
Student membership starts from only £1 a month, with members receiving a whole host of additional benefits, including reduced registration to events, access to prizes and bursaries, a printed copy of BNA Bulletin and free membership of Federation of European Neuroscience Societies (FENS).








 Brain Insights
Brain Insights Cannabis is the most prevalent illegal substance used in the world today (1) and represents a drug class with use spanning diverse cultural, recreational and medicinal settings. Like many illicit compounds, its reputation is embodied by themes of criminality and poor mental health. However, understanding the endogenous and exogenous applications of cannabinoids (molecules primarily interacting with cannabinoid receptors) has the capacity to drastically aid a current mental health crisis - depression. At present, the utility of cannabinoids for treating conditions such as depression is in-optimal, as we will explore in the case of the recent approval and subsequent removal of the antiobesity drug, rimonabant.
Cannabis is the most prevalent illegal substance used in the world today (1) and represents a drug class with use spanning diverse cultural, recreational and medicinal settings. Like many illicit compounds, its reputation is embodied by themes of criminality and poor mental health. However, understanding the endogenous and exogenous applications of cannabinoids (molecules primarily interacting with cannabinoid receptors) has the capacity to drastically aid a current mental health crisis - depression. At present, the utility of cannabinoids for treating conditions such as depression is in-optimal, as we will explore in the case of the recent approval and subsequent removal of the antiobesity drug, rimonabant.
