Place your votes: BNA Council & Committee elections
22nd July 2024
28th Feb 2022

 Brain Insights is the dedicated student section published in the British Neuroscience Association (BNA) Bulletin. It represents the voice of the BNA student: written by students for students. In this student article,
Brain Insights is the dedicated student section published in the British Neuroscience Association (BNA) Bulletin. It represents the voice of the BNA student: written by students for students. In this student article,
Natalia Romanow, MSci student at King’s College London, (right) explores whether schizophrenia is a pathology of synapses or homeostasis.
Writing for Brain Insights is a members-only benefit. If you're not already a member you can join here from just £1 a month.
In 1899, German psychiatrist Emil Kraepelin first described a mysterious disorder, known today as schizophrenia (1). In the 122 years since, great scientific and technological advances have been made, from the lunar landing of 1969 to the ground-breaking cloning of Dolly the sheep in 1996. Yet, our understanding of the aetiology of many psychiatric and affective disorders remains incomplete, including schizophrenia.
Schizophrenia (SCZ) is a largely heterogenous disorder range of symptoms, from positive (hallucinations. delusions) to negative (apathy, social withdrawal) . Many scientists believe SCZ to be a disease of the synapse – the site of communication between neurons (2). Post-mortem studies of confirmed SCZ diagnoses have demonstrated a significant reduction in the number and size of specialized protrusions in the post-synaptic cells known as dendritic spines (3). As shown in Figure 1, there are fewer dendritic spines on cortical neurons in the brains of SCZ patients when compared to healthy controls. Moreover, Positron Emission Tomography (PET) scans revealed SCZ patients to have reduced levels of the synaptic vesicle glycoprotein 2A (SV2A) (4). SV2A is a chronic exposure to haloperidol and olanzapine.
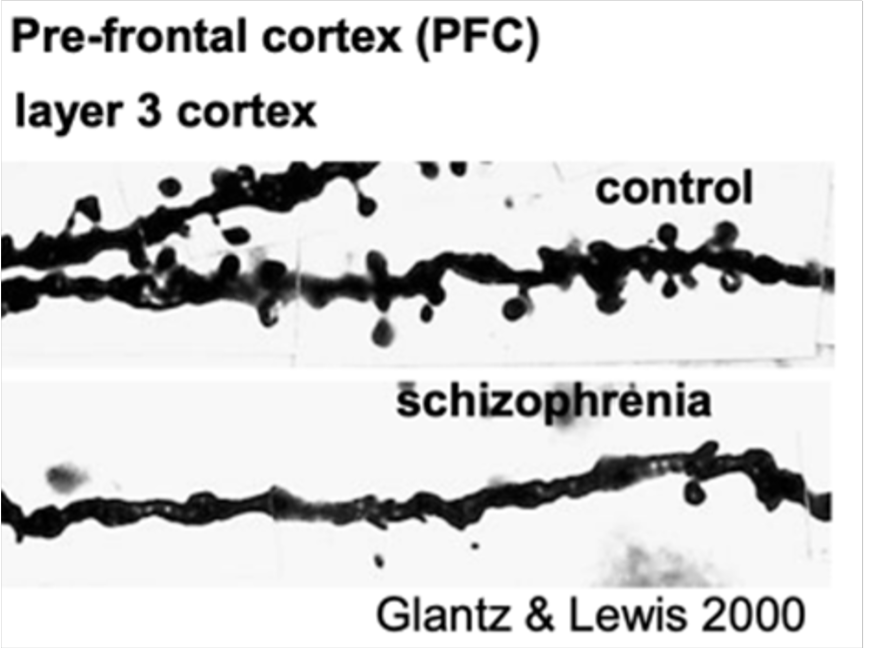
Figure 1 (above). Dendritic pathology in schizophrenia. Glantz & Lewis, 2000
The view that synaptic protein folding is involved associated with schizophrenia is one that has gained traction amongst academics and clinicians alike. Professor Ian Everall of King’s College London for instance, reinforces this perspective: “I think it (schizophrenia) is all about proteins.”. For a protein to perform its intended function, it needs to be folded correctly, conferring appropriate tertiary and quaternary structures. When this process fails, the ubiquitin-proteasome system (UPS) works to control functional protein stability by degrading misfolded proteins to prevent protein aggregation, acting as part of a mechanism known as proteostasis. Proteostasis is a protein specific form of homeostasis which seeks to maintain protein stability, which akin to other forms of homeostasis, promotes regulation of cellular (and by effect macroscopic) structure and function. The dysfunction of proteostatic mechanisms such as this can be associated with pathology, as discussed above with respect to schizophrenia.
Whilst proteostasis can become dysfunctional in numerous neurodegenerative diseases, there is little information on how it manifests in schizophrenia. Professor Everall was part of one of the few studies to have measured the ubiquitinated protein levels in the blood and brains of SCZ patients (5). The researchers detected a considerable decrease in ubiquitination in the blood and the brain. In other words, both the blood and the brain of schizophrenia patients show an abnormal ubiquitinated protein formation. This build-up of proteins can be linked to the neurotoxicity or pathogenesis of schizophrenia. Such findings hold great promise given that protein ubiquination may serve as a potential prognostic tool for identifying schizophrenic risk in treatment-resistant individuals, who comprise approximately 30% of schizophrenia patients (6).
Could dysfunctional proteostasis impact synapses in SCZ? So far, laboratories have been mostly interested in local protein synthesis, as opposed to protein degradation. When a synapse is activated, a process called long-term potentiation (LTP) causes the dendritic spines to increase in size and more AMPA receptors (AMPAR) emerge. This leads to the persistent strengthening of synapses, producing a long-lasting increase in signal transmission between the two activated neurons. Where do those AMPA receptors come from? There might be a reserve of AMPARs in the cytosol which are then trafficked to the synapse following LTP, without which the potentiation of other synapses could not propagate further. Proteostasis provides the necessary balance. Many questions in this area remain to be answered. For instance, do proinflammatory cytokines exacerbate or ameliorate such a mechanism? Future work will likely need to explore these pathways on the road to improving our understanding of schizophrenia.
References
1. Falkai, P., Rossner, M., Schulze, T. et al. Kraepelin revisited: schizophrenia from degeneration to failed regeneration. Mol Psychiatry. 2015; 20: 671–676 Available from: doi: 10.1038/mp.2015.35
2. Momtazmanesh, S., Zare-Shahabadi, A., & Rezaei, N. Cytokine Alterations in Schizophrenia: An Updated Review. Frontiers in Psychiatry. 2019; 10: 892. Available from: doi: 10.3389/fpsyt.2019.00892
3. Glantz, L. A., & Lewis, D. A. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Archives of General Psychiatry. 2000; 57(1); 65–73. Available from: doi: 10.1001/archpsyc.57.1.65
4. Onwordi, E.C., Halff, E.F., Whitehurst, T. et al. Synaptic density marker SV2A is reduced in schizophrenia patients and unaffected by antipsychotics in rats. Nat Commun. 2020; 11(246). Available from: doi: 10.1038/s41467-019-14122-0
5. Bousman, C.A., Luza, S., Mancuso, S.G. et al. Elevated ubiquitinated proteins in brain and blood of individuals with schizophrenia. Sci Rep. 2019; 9, 2307. Available from: doi: 10.1038/s41598-019-38490-1
6. Kane, J.M., Agid, O., Baldwin, M.L., et al. Clinical guidance on the identification and management of treatment-resistant schizophrenia. J Clin Psychiatry. 2019; 80(2): 18com12123.
Brain Insights welcomes contributions from our undergraduate and postgraduate students, Career Starter and early ECR members who are interested in writing.
To express an interest in writing an article, please contact one of our editors below:
Writing for Brain Insights is now a members' only benefit. If you're not already a member you can join here.
Student membership starts from only £1 a month, with members receiving a whole host of additional benefits, including reduced registration to events, access to prizes and bursaries, a printed copy of BNA Bulletin and free membership of Federation of European Neuroscience Societies (FENS).