Place your votes: BNA Council & Committee elections
22nd July 2024
In addition to the print editions of Bright Brains, here you can find extended online content. All articles are by and for our student and early-career members.
Bright Brains has now been succeeded by Brain Insights, the current student newsletter publication. If you’re interested in contributing to Bright Insights online or in print, please email Alex Campbell (alex.campbell@bna.org.uk).
Contents |
Editor-In-Chief:
Jayanthiny Kangatharan, PhD
Editors:
Stephanie Baker, PhD, Inês Barreiros, Francesco Berti, Deannah Blackely, Jeremy Chabros, Jack Cooper, Nerissa Culi, Aisha Islam, Jayanthiny Kangatharan, PhD, Paulina Pokorska, Laura Riggall, Marco Travaglio
By Marco Travaglio, PhD student in Neuroscience, University of Cambridge
Motivation is a hard thing to get. Whereas at home, at school or at work, most of us will have been in that situation in which we know exactly what to do but we just lack the mental power to do it. We live in a world where the constant hype and bustle has made us accustomed to make hasty decisions, to inflate our working hours to meet our ever-growing ambitions, and to feel just like another cog in the machine of society. All of this can critically undermine our willpower, creating unprecedented gaps in our motivation to learn, study and work to a standard that our society defines appropriate. Without motivation, everything can seem inherently absurd and pointless. This invigorating force enables you to act, whether it is to get out of bed or to sign the much-awaited contract that will shape the rest of your career.
In recent years, there has been increasing interest towards understanding the biological mechanisms that fuel our motivation. Dopamine has emerged as a key chemical involved in almost every aspect of motivation and it is now clear that motivation can be tracked down to a few, circumscribed regions of the brain. Like every other neurotransmitter, dopamine conveys its signal by passing from one neurone to the next, but which route does dopamine take in the brain to energize your thoughts? It took many years of hard work and willpower (thus much dopamine consumed along the way) to identify what scientists now call the ‘mesolimbic pathway’, otherwise known as a neuronal circuit that connects the middle of the brain (midbrain) to the its outermost region (cerebral cortex). Dopaminergic neurones originate from the Ventral Tegmental Area (VTA), from where they project to the nucleus accumbens as well as other ‘limbic’ regions, including the hippocampus, amygdala, prefrontal cortex and septum. While a nuanced picture of how these regions interact with each other is still missing, experts agree that dopamine is what tips the balance between spending an afternoon on your couch ruminating over your lack of exercise and going to the gym.
The earliest experiments linking dopamine to motivation trace their roots back to the early 50’s when a researcher called James Olds discovered that electrical stimulation of the middle of the brain would cause rats to repeat a given behaviour [1]. The idea that this effect could be mediated by dopamine was later formalized by Roy Wise, whose experiments demonstrated that low levels of dopamine in an animal’s brain attenuate its motivation to act and execute well-learned behaviours to obtain food, water or sexual contact [2]. These findings were thought to suggest that dopamine regulates our ability to perceive pleasure and reward, a theory that rapidly found its way into the popular culture. However, it was not until recently that this idea was overturned to show that dopamine activity may actually be related to human motivation instead. In a series of experiments, a group of researchers led by John Salamone at The University of Connecticut discovered that when animals are given a choice between a high-value reward and a low-value-reward that can be achieved through different levels of effort, the difference in their choice may lie in the brain’s dopamine levels. Artificial manipulation of dopamine content showed that animals with lower levels of dopamine opt for the low-value, easier-to-obtain reward whereas animals with higher levels of brain dopamine would show increased motivation to work harder to obtain the high-value reward [3].
While these studies offer valuable insights into how dopamine gets you out of bed, it is important to note that most studies were carried out in rodents. In the attempt to expand these findings to other species, scientists at Standford University thought to measure dopamine levels in some of the most laborious animals on the planet: ants. A new study published in the Journal of Science indicates that when ants are given dopamine, they exhibit increased motivation to leave their nest and to start their foraging activity than ants given a control substance [4]. The authors of this study interpreted these findings as evidence that dopamine can influence a forager’s evaluation of environmental stimuli as well as of its own physiological state. Essentially, increased dopamine signalling may alter the animal’s perception of its own physiological readiness to forage by overriding negative cues that regulate this evolutionary conserved behaviour. Long story short, dopamine juices you up. But where does dopamine act in the brain to exert its effects? Consensus revolves around the idea that when dopamine travels from the VTA along the mesolimbic pathway, it bumps into a structure called ‘nucleus accumbens’. You can think of this as your personal coach. High levels of dopamine in this area will encourage you to tick every box of your to-do list whereas low levels will leave you sprawling in your bed.
So what can we do to improve our motivation? Is it enough to crank up our brain’s dopamine levels? Quite the opposite. Scientists at Vanderbilt University demonstrated that while high levels of dopamine in known motivational centres can enhance your willingness to work hard for a reward, high levels of dopamine are also found in the brain of people that show reduced motivation to work [5]. Just in a different part of the brain. This finding suggests that while enhancing your brain’s dopamine content can have positive effects on your motivation, the effects could vary depending on where dopamine is increased. Similarly, a new study published in Neuron indicates that dopamine is also released into the nucleus accumbens in response to unpleasurable experiences, adding to the notion that this transmitter can function as a double-edged sword in the brain [6]. How can we hack this system? Train your brain. Researchers suggest that our brain can be trained to create the right dopamine environment and one way to do this is by anticipating the reward. Visualise the completion of a project and embrace the perceived reward from completing the task. Using real-time brain imaging techniques, neuroscientists have found that reward information is processed in an area of your brain called the prefrontal cortex and that this area interacts directly with the nucleus accumbens and VTA to trigger motivated behaviour (7). What this means is that anticipated reward can directly influence your willingness to work by activating the very key brain motivation centres.
But ultimately, while neuroscience certainly helps to hack our biological constraints, we shall not forget that old-school self-discipline and determination are two of the most powerful forces to boost your motivation. Much research is needed to fully understand the complex biological mechanisms that generate and maintain our willpower but, in the meantime, passion and perseverance into whatever you are doing will probably get your dopamine flowing. The rest will follow.
References
1. Olds, J., (1956). Runway and maze behavior controlled by basomedial forebrain stimulation in the rat, Journal of Comparative and Physiological Psychology, 49:507–12.
2. Wise R.A. and Schwartz H.V, (1981). Pimozide attenuates acquisition of lever pressing for food in rats. Pharmacology, Biochemistry and Behaviour, 15, 655–656 (1981).
3. Salamone J.D., (2009). Dopamine, effort, and decision making: theoretical comment on Bardgett et al., Behavioural Neuroscience, 123(2):463-7.
4. Friedman D.A., Pilko A., Skowronska-Krawczyk D., et al., (2018). The role of dopamine in the collective regulation of foraging in harvester ants, iScience, 8:283-294.
5. Treadway MT, Buckholtz JW, Cowan RL, et al., (2012) Dopaminergic mechanisms of individual differences in human effort-based decision-making. Journal of Neuroscience, 32(18): 6170–6176.
6. de Jong J.W., Afjei S.A., Dorocic I.P. et al., (2018). A neural circuit mechanism for encoding aversive stimuli in the mesolimbic dopamine system, Neuron, doi.org/10.1016/j.neuron.2018.11.005
7. Ballard I.C., Murty V.P., Carter R.M., et al (2011). Dorsolateral prefrontal cortex drives mesolimbic dopaminergic regions to initiate motivated behaviour, The Journal of neuroscience : the official journal of the Society for Neuroscience, 31(28), 10340-6.
By Marco Travaglio, PhD student in Neuroscience, University of Cambridge
Historians used to call it a ‘cradle of civilization’ but for many scientists today the real value of the Mediterranean basin does not lie in its contribution to history. Home to one of the largest concentration of built heritage sites, these lands have often been celebrated for their mystic healing power, offering long-sought shelter to harassed minds, mending the damaged lives of writers and artists from all over the world. Whilst the natural landscapes and lifestyle of this region are indisputably linked to the profound sense of contentment that tourists often experience when entering it, an important part of the appeal arises from its much celebrated cuisine. Indeed, the Mediterranean diet (MeDi) has long attracted extensive media attention due to a growing international taste for the cooking of the lands bordering the inland sea. However, this fascination for all edibles Italian and Mediterranean is also sparked by accumulating evidence of its wide-ranging benefits on the body and, more recently, on the brain.
Indeed, evidence suggests that feeding your brain with the plant-based diet of the olive-growing Mediterranean regions may reduce the risk of cognitive problems, effectively making you live a longer and healthier life. But what does MeDi consist of? According to Ancel Keys, one of the first scientists to champion the benefits of this diet, MeDi involves a high intake of fruit, vegetables, nuts, cereals and olive oil, limited amount of meat and moderate amount of fish, dairy products and wine [1]. This dietary pattern was originally identified in Greece and Southern Italy and thus, it is little surprise that one of the first studies investigating the effects of MeDi on brain health took place in Italy almost 30 years ago. As part of a broader investigation into the prevalence of several diseases in older adults, researchers at The University of Bari discovered that individual elements of the MeDi are protective against cognitive decline [2]. Their investigation involved the use of a standard clinical test to assess cognitive decline every 3 to 5 years and a dedicated questionnaire to evaluate participants’ dietary choices. This pioneering work demonstrated that a large intake of monounsaturated fatty acids (MUFA), abundant in olive oil, is protective against cognitive decline.
Encouraged by these intriguing results, scientist Valls-Pedret and colleagues at the Barcelona’s Institut d’Investigacions Biomèdiques August Pi Sunyer embarked on the first-of-its-kind clinical trial looking at the association between MeDi and cognitive function in older individuals. Seeking to confirm previous findings, the authors of this study compared the effects of a MeDi supplemented with either extra-virgin olive oil or mixed nuts with a control diet that consisted of advice to reduce dietary fat [3]. Results were striking. After several years of data (and food) crunching, researchers demonstrated that participants allocated the Mediterranean-style diets displayed improved learning and memory whereas those allocated a normal diet showed signs of cognitive decline. Moreover, later analysis revealed that differences between groups could not be attributed to subjects’ gender, age or education level indicating that the effect was likely driven by their dietary choice.
So how does MeDi exert its effect on the brain? While various attempts have been made to explain its beneficial effects, mechanisms remain nebulous at best. Most experts suggest that the mysterious effects of MeDi could stem from the abundance of anti-oxidant agents that it provides. Oxidative stress is often cited as major cause of cell damage in several age-related brain conditions and is caused by the accumulation of toxic compounds in the brain called free radicals. With time, excessive accumulation of free radicals can lead to the irreversible loss of brain cells, which can profoundly affect learning and memory. Complex substances with important anti-oxidant properties such as olive oil, wine, nuts, fruits and vegetables have been found in high concentrations in MeDi, suggesting that these substances may reduce oxidative processes in the brain, preserving normal brain function. In addition, people observing a MeDi pattern have been reported to display reduced inflammation in their brain, shedding light on another potential mechanism explaining the protective effect of MeDi. Chronic inflammation of brain cells has also been associated with a number of age-related brain conditions, as the build-up of inflammatory substances in the brain appears to facilitate the accumulation of toxic proteins which, with time, lead to cell death in a number of diseases.
Now, since brain cells are continuously attacked by oxidative stress and inflammation as we get older, it should come as no surprise that human brains shrink with age due to cell loss. However, high intake of anti-oxidant and anti-inflammatory substances should in theory delay or stop this shrinkage by a considerable amount. This idea was recently put to test by neuroscientist Michelle Luciano, who last year analysed changes in brain structure in hundreds of individuals in their 70s. Their results demonstrated that the brains of those who adhered most closely to a typical MeDi shrank less than those of individuals that did not adhere to the same diet [4]. As tantalizing as it sounds, Dr Luciano specified that differences in thinking and memory between groups were not explored in this study, making it difficult to draw any conclusion on the effect of this well-balanced diet on brain function. In addition, a recent Italian study found that the benefits arising from MeDi are only evident in people with higher income or better education, putting into question the very notion that MeDi benefits everyone. Indeed, the same study suggested that only people with a household income over £35.000 (~$45.700) could benefit from this diet, whereas no benefits were found in less advantaged groups [5].
In conclusion, while mounting research points to the beneficial effects of MeDi on brain health, more research is needed to prove its effects. Most studies suggest a link between this diet and brain function but little is known about the mechanisms that drive its effects on the brain. In addition, defining minimum quantities of recommended foods remains a priority. Researchers agree that there are lots of benefits and no known downside to following a healthy diet. However, whilst adding olive oil and mixed nuts to your diet may benefit your brain cells, using excessive amounts will only make you gain weight. So… don’t go nuts!
References
1. Keys A., Aravanis C., Blackburn H.W., Van Buchem F.S., Buzina R.,Djordjevic B.D., Dontas A.S., Fidanza F., Karvonen M.J., Kimura N., et al., (1966). Epidemiological studies related to coronary heart disease: characteris-tics of men aged 40–59 in seven countries, Acta medica scandinavica Supplements, 460:1–392.
2. Solfrizzi V., Panza F., Torres F., Mastroianni F., Del Parigi A., Venezia A., Capurso A., (1999). High monounsaturated fatty acids intake protects against age-related cognitive decline, Neurology, 52(8): 1563- 1569.
3. Valls-Pedret C., Sala-Vila A., Serra-Mir M., Corella D., de la Torre R., Martínez-González M.Á., Martinez-Lapiscina E.H., Fitó M., Pérez-Heras A., Salas-Salvadó J., Estruch R., Ros E., (2015). Mediterranean diet and age-related cognitive decline: A randomized clinical trial, JAMA internal medicine, 175: 1094–1103.
4. Luciano M, Corley J, Cox SR, Valdés Hernández MC, Craig LCA,Dickie DA, Karama S, McNeill GM, Bastin ME, Wardlaw JM, et al., (2017). Mediterranean-type diet and brain structural change from 73 to 76 years in a Scottish cohort, Neurology, 88: 449–55.
5. Bonaccio M., Di Castelnuovo A., Pounis G., Costanzo S., Persichillo M., Cerletti C., Donati M.B., de Gaetano G., Iacoviello L., (2017). Study Investigators High adherence to the Mediterranean diet is associated with cardiovascular protection in higher but not in lower socioeconomic groups: Prospective findings from the Moli-sani study, International journal of epidemiology, 46(5): 1478-1487.
By Rachel Jones, Graduate student in Neuroscience, University of Leeds
Environmental awareness surrounding climate change has become an increasingly common topic of discussion amongst politicians, the media and general public alike. The effects of air pollution on respiratory and cardiovascular health are well-documented, accounting for a substantial proportion of deaths worldwide due to lung cancer, ischemic heart disease and stroke [1]. Currently, the effects of air pollution on the central nervous system (CNS) are being investigated, in particular its involvement in the development of Alzheimer’s disease (AD).
One of the first examples of a link between air pollution exposure and cognitive decline, the primary characteristic of AD, was in 2008 in Mexico City. Healthy children in Mexico City with no known risk factors for cognitive dysfunction exhibited prefrontal white matter hyperintense lesions and significant deficits in cognitive tasks, compared with their clean air counterparts [2]. These results gained traction and inspired a range of subsequent studies reporting similar findings. Adults exposed to long-term high levels of air pollution tend to show significant deficits in verbal memory, attention [3] and episodic memory [4] compared to less exposed adults. The trend of results even suggested that each 10 μg/m3 increment increase in particulate matter (PM, particles suspended in air pollution) concentration that adults were exposed to was equivalent to cognitively aging approximately 2 years [3].
Thus, a relationship emerged between cognitive decline and air pollution. Researchers consequently hypothesised that long-term PM exposure could be a risk factor for the development of AD. Incomplete knowledge of AD disease progression paired with the ability to mediate environmental risk factors (unlike genetic risk factors) provided motivation to take research further. Abnormal Aβ deposition [5], hippocampal neuronal atrophy [6] and upregulation of proinflammatory mediators [7] are present in the brains of both highly exposed humans and animals. These are all well-established hallmarks of AD. Most recently, a study carried out in London reported a positive exposure-response relationship between air pollution and incidence of AD diagnosis in adults with no recorded history of dementia [8], unexplained by confounding factors.
Current research is rapidly opening up to include multiple CNS effects including white matter injury, ADHD and depression [9, 10]. In particular, the detrimental long-term effects of air pollution on the developing brain are coming to light [9]. Our aging population will naturally result in increased financial and emotional burdens on society, stemming from age-related diseases including AD. In a ‘two-birds-one-stone’ approach, being mindful of our environmental impact and reducing levels of air pollution could help mitigate these burdens. Further research is imperative to obtain concrete evidence of links between air pollution and AD, potentially influencing environmental policy change worldwide.
References
1. Mannucci P & Franchini M (2017) Health Effects of Ambient Air Pollution in Developing Countries. International Journal of Environmental Research and Public Health 14(9):1048.
2. Calderón-Garcidueñas L, et al. (2008) Air pollution, cognitive deficits and brain abnormalities: a pilot study with children and dogs. Brain and Cognition 68(2):117-27.
3. Weuve J, et al. (2012) Exposure to Particulate Air Pollution and Cognitive Decline in Older Women. Archives of Internal Medicine 172(3):219-227.
4. Ailshire J & Crimmins E (2014) Fine Particulate Matter Air Pollution and Cognitive Function Among Older US Adults. American Journal of Epidemiology 180(4):359-366.
5. Calderón-Garcidueñas L, et al. (2004) Brain inflammation and Alzheimer's-like pathology in individuals exposed to severe air pollution. Toxicologic Pathology 32(6):650-658.
6. Cacciottolo M, et al. (2017) Particulate air pollutants, APOE alleles and their contributions to cognitive impairment in older women and to amyloidogenesis in experimental models. Translational Psychiatry 7(1):e1022.
7. Zheng X, et al. (2019) Gestational Exposure to Particulate Matter 2.5 (PM2.5) Leads to Spatial Memory Dysfunction and Neurodevelopmental Impairment in Hippocampus of Mice Offspring. Frontiers in Neuroscience 12:1000.
8. Carey I, et al. (2018) Are noise and air pollution related to the incidence of dementia? A cohort study in London, England. BMJ Open 8(9):e022404.
9. Sram R, et al. (2017) The impact of air pollution to central nervous system in children and adults. Neuro Endocrinology Letters 38(6):389-396.
10. Babadjouni R, et al. (2017) Clinical Effects of Air Pollution on the Central Nervous System. A Review. Journal of Clinical Neuroscience 43:16-24.
By Marta Blanco-Pozo, PhD student in Neuroscience, University of Oxford
From making code and data available to publishing in open access journals, a great effort is currently being made to promote Open Science and to make research accessible to everyone. Major funding bodies, including the Wellcome Trust, are now updating their policies to align with Plan S, which is “an initiative to make full and immediate Open Access to research publications a reality”. This means that from 2021, all scientific research findings must be published in Open Access journals or platforms. But do we know how this will affect both senior and early career researchers?
To address this question, the Cortex Club –the Oxford University Neuroscience Society– organised a panel discussion on Open Science and Open Access. We invited Elisa de Ranieri, editor in Nature Communications, John Inglis, co-founder of bioRxiv and medRxiv and currently executive director in Cold Spring Harbor Laboratory Press, Sally Rumsey, Head of Scholarly Communications & Research Data Management in the Bodleian Library, and Mark Patterson, executive director of eLife.
We started by discussing how journals, institutions and repositories are improving the way research is being published and shared, how subscription fees are increasing at a higher rate than university funds, and how universities and institutions could support publishing in open access journals. Then, the focus of the debate shifted to the impact that open access might have on researchers’ careers.
Some institutions like Oxford have signed the San Francisco Declaration on Research Assessment (DORA), which states that the impact factor from journals cannot be used to assess quality research and cannot be used in the hiring process. However, this declaration has not been signed by all research institutions, and a recent meta-analysis [1] showed that 40% of research universities in the United States and Canada explicitly mention that journal impact factor can be used for tenure and promotion evaluations. Does this mean that publishing in open access journals with lower impact factor rather than top renowned closed journals could jeopardise early career researchers’ future or make it difficult for more senior researchers to keep their positions?
All of the panellists agreed on the complexity of this issue, and it did not seem that there is a solution yet to address this problem. Moreover, a way to assess research quality aside from journals impact factor has not been established yet. In brief, the research community needs to continue discussing, and find new ways of addressing these issues. Initiatives like Plan S are certainly changing research publishing culture. However, the way this will affect researchers at different career levels and institutions is still to be determined.
References
1. McKiernan EC, Schimanski LA, Muñoz Nieves C, Matthias L, Niles MT, Alperin JP (2019) Use of the Journal Impact Factor in academic review, promotion, and tenure evaluations. Elife 8.
By Ryan Stanyard, PhD student in Neuroscience, King’s College London
In recent years, both Neuroscience and the Psychological Sciences have witnessed a call for a robust framework to provide online materials that better enable scrutiny of research. This will help guard both the validity of financial investment to research and mark a stand against publication bias and credibility inflation [1]. Previous media scrutiny has ousted pioneers in Psychology, discredited for fabricating data, using convoluted methodologies to mask the absence of true results and misleading readers [2, 12].
Far more commonly but less dramatically and undermining for the public, a paucity of published materials and data has contributed to what has been termed the ‘Replication Crisis’. This term was popularised largely in the Psychological literature but it is also relevant to Neuroscience and the Medical sciences. Neuroscientists have endeavoured to provide better access to both raw and processed data in addition to other research materials. Similarly, neuroscience has begun to introduce Registered Reports in line with new guidelines [3], a framework common in international and multi-centre protocols for imaging or genomic studies, amongst others. Nonetheless, the transition to replicable science is not progressing without difficulty [4].
In 2019 in which the British Neuroscience Festival (Dublin) [5] and the meeting of the Organisation of Human Brain Mapping (Rome) [6] have been celebrated, we have also seen papers exploring themes such as neural oscillations in artificially re-perfused porcine tissue (7) gracing the likes of Nature. Yet, equal strides in fundamental changes to research and publication structure are under way. The introduction of the Open Science (OSF) [8] and Registered Reports frameworks is becoming lucrative to many stakeholders. These platforms are enabling researchers to critically review changes to methods and flag gaps in planned analyses. Alleviating the focus from high-impact publishing in the wake of the Research Excellence Framework [9] and the archaic pyramid scheme in academic advancement, this new approach provides better means of appraising bids for funding high-expenditure research projects and acknowledges the merit of peer-scrutiny [10].
Adjustment to new terms in this arena may be daunting. There may be questions as to how will early-career researchers engage with pre-published or pre-print materials or how they will convince principal investigators opposing the movement. OSF advocates for more transparent, supportive scrutiny of peer-reviewed literature prior to, during and following formal publication. Ultimately, this gives more credibility to Neuroscience research [11]. For example, flagging weakly structured projects (e.g. unclear or aberrant methods) may facilitate better allocation of funding and resources between projects, and alleviate controversy in literature where acquisition and analysis methods may differ between groups or institutions.
References
1. Rousselet, A. G., et al., (2019). Promoting and supporting credibility in neuroscience. Brain and Neuroscience Advances 3, 1–4.
2, Callaway, E. (2012) Danish neuroscientist challenges fraud findings. Nature. (DOI:10.1038/nature.2012.11146, Accessed: 8th October 2019)
3, The Royal Society (2019). Registered Reports. Retrieved from: https://royalsocietypublishing.org/rsos/registered-reports [Accessed: 8th October 2019]
4. Ayris, P., de San Román, A. L., Maes, K., Labastida, I. (2018). Open Science and its role in universities: a roadmap for cultural change. Retrieved from: https://www.leru.org/publications/open-science-and-its-role-in-universities-a-roadmap-for-cultural-change# [Accessed: 8th October 2019]
5. British Neuroscience Association (2019). Festival of Neuroscience. Retrieved :https://www.bna.org.uk/mediacentre/events/bna2019-festival-of-neuroscience/ [Accessed: 8th October 2019]
6. Organisation for Human Brain Mapping (2019). 2019 OHBM Annual Meeting. Retrieved: https://www.humanbrainmapping.org/i4a/pages/index.cfm?pageID=3882&activateFull=true [Accessed: 8th October 2019]
7. Vrselja, Z. et al., (2019) Restoration of brain circulation and cellular functions hours post-mortem. Nature 568(7752), 336–343.
8. The Open Science Framework (2019). Retrieved from: https://cos.io/our-products/osf/ [Accessed: 8th October 2019]
9. Research Excellence Framework (2019) Retrieved from: https://www.ref.ac.uk/about/what-is-the-ref/ [Accessed: 8th October 2019]
10. David Mellor (2019). An Introduction to Registered Reports for the research funders community. Retrieved from: https://osf.io/nav8d/ [Accessed: 8th October 2019]
11. Mellor et al., (2019). Supplemental materials for preprint: Towards minimum reporting standards for life scientists. Retrieved from: https://osf.io/2k3va/ [Accessed: 8th October 2019]
12. Using Science to Sniff Out Science That’s Too Good To Be True. Retrieved from: http://blogs.discovermagazine.com/crux/2012/09/19/when-science-is-too-good-to-be-true/#.XZCeekZKgtI [Accessed: 8th October]
By Ryan Stanyard, PhD student in Neuroscience, King’s College London
In conventional structural (sMRI) and functional MRI (fMRI) [1], images acquisition is led by radiographers (or researchers in some institutes). Subjects are scanned according to study protocols, adjusting parameters for the size of the subjects’ brain volume. A series of pre-planned scans are to be completed within a confined scanning window, which in many studies is typically ~1hr. Efficient acquisitions often employ parallel imaging [2] techniques (using spatial localisation techniques to reduce phase encoding steps during acquisition, effectively reducing scan times). These technologies can be co-utilised alongside a Bayesian Optimisation approach [3, 4, 5]; rather than scanning an entire subjects’ brain/‘volume’ according to protocol, subjects are scanned at increasing resolutions in sub-sampled regions of interest or indeed the whole brain. Next, this individuals’ scan is compared in real-time against a database of normative typically developing healthy controls, matching for resolution, age and other stratifiers of interest (e.g. sex, disease severity etc.), yielding a dynamic and flexible approach. By comparing in-scan subjects against normative populations for given tasks, the algorithm can derive which tasks/areas an individual is most ‘different’ from relative to a normative sample, guiding subsequent acquisitions.
This is useful for (a) individuals who have low tolerance for MRI (e.g. claustrophobics, hyper-anxious, physically/mentally disabled, ADHD) do not need to undergo less important scans and can complete either more relevant runs of a task/paradigm or finish early, (b) focusing scanning on tasks/areas of interest and (c) in totality, delivering lucrative cost savings. Given conventional imaging costs ~£400-600 per hour (equiv. in U.S.) [6], whether commercially, for research or clinically, shorter scan times could potentially deliver billions (£) per annum in savings for bodies such as the NHS. Conversely, scans from conventional imaging are routinely anonymised (personal data is redacted) and so can be used by other researchers to bolster modelling normative distributions if technical specifications match their own sampling strategy.
Further applications include stratifying groups and testing cognitive task elicitation of neurocircuitry (which tasks activate which brain networks); assuming some variable parameters of task X evokes brain response in circuits Y and Z. Responses may be associated with mental processes or functions distinct from another task. An example of this may be where a visual task presents slow-moving high-contrast figures in the foreground which the user generally focuses on, activating visual circuits distinct from another task where the user has to pay attention to a fast moving stimulus (akin to ‘oddball’ tasks). Dissecting key variations in task performance and task-relative neuroactivation at both the individual and group-levels (e.g. ASD vs controls) [7, 8] may provide novel biomarkers focal to delivering personalised medical therapeutics.
References
1. Glover, G. H. (2011) Overview of functional magnetic resonance imaging. Neurosurgery Clinics of North America 22 (2) 133–139.
2. Deshmane, A., Gulani, V., Griswold, M.A., and Seiberlich, N. (2012) Parallel MR imaging. Journal of Magnetic Resonance Imaging 36 (1) 55–72.
3. The Royal Society (2019). Registered Reports. Retrieved from: 29th September, 2019. https://royalsocietypublishing.org/rsos/registered-reports
4. Lorenz R. et al. (2016). The Automatic Neuroscientist: A framework for optimizing experimental design with closed-loop real-time fMRI. Neuroimage 129, 320-334.
5. Lancaster J. Lorenz, R., Leech, R., and Cole, J. H. (2018) Bayesian optimization for neuroimaging pre-processing in brain age classification and prediction. Frontiers in Aging Neuroscience 10 (28), 1–10.
6. Camprodon, J. A. & Stern, T. A. (2013) Selecting neuroimaging techniques: A review for the clinician. Primary Care Companion for CNS Disorders 15 (4).
7. Lorenz, R. et al. (2018) Dissociating frontoparietal brain networks with neuroadaptive Bayesian optimization. Nature Communications 9 (1), 1227.
8. Organisation for Human Brain Mapping (2019). 2019 OHBM Annual Meeting. Retrieved: 29th September 2019: https://www.humanbrainmapping.org/i4a/pages/index.cfm?pageID=3882&activateFu ll=true
By Andreea Pantiru, PhD student in Neuroscience, University of Leeds
Fear is an emotional experience deeply ingrained in evolution to foster survival and enable avoidance of potentially harmful conditions. However, fear may become dysfunctional and result in anxiety and trauma-related disorders, such as post-traumatic stress disorder (PTSD). PTSD is a debilitating mental health condition and exposure therapy is the main approach to combat PTSD. However, 40% of patients fail to respond to this therapy [1]. Fear encoding is not fully understood; therefore, development of better treatments requires an improved comprehension of how fear is processed in the brain. Fear extinction is a learning mechanism, which manifests in a decline in fear responses towards a stimulus that was previously eliciting fear. Extinction is essential for understanding fear-related disorders as it is the process upon which exposure therapy is based. But how can we target this extinction process?
Fear-conditioning is a well-established technique, which helped identify the amygdala as a key structure of the brain involved in the formation, consolidation and extinction of fearful memories [2]. This learning paradigm represents the coupling of an event, the conditioned stimulus (CS), with an aversive event, the unconditioned stimulus (US). Even one CS–UC pairing was shown to be sufficient to elicit a fear response to CS alone [2]. CS-dependent fear can be diminished through subsequent repeated and unreinforced re-exposure to the CS, which leads to fear extinction. Enhancing fear extinction could potentially ameliorate disorders, such as PTSD, by inhibiting the intrusive recollection of traumatic experiences [3].
Basolateral nucleus of amygdala (BLA) contains two electrophysiologically distinct populations neurons: one which is activated during acquisition and one activated during extinction of fear [4]. Research revealed that thymus cell antigen 1 (Thy1) is distinctively expressed in extinction cells, which mark a population of glutamatergic neurons within the BLA [5, 6]. Activation of Thy1 neurons in the BLA blocked the transmission of fear-related signals to downstream areas, important in regulating physiological and behavioural consequences following fear exposure [5], and revealed a role of Thy1-expressing BLA neurons in fear circuitry. Behavioural analysis displayed that optogenetic inhibition of BLA Thy1-expressing neurons during fear conditioning/extinction results in increased fear response, while chemogenetic activation of these neurons reduces fear-related behaviours in mice [6], highlighting the importance Thy1-neurons have in extinction [6].
Overall, these studies emphasize the significance Thy1-expressing BLA neurons play in mediating fear inhibition and providing insight into a new potential target for fear disorders, such as PTSD. A better understanding of how fear is encoded will ultimately lead to efficient biologically driven approaches for treatment and prevention of fear-related disorders.
References
1. Singewald N, Schmuckermair C, Whittle N, Holmes A, & Ressler KJ (2015) Pharmacology of cognitive enhancers for exposure-based therapy of fear, anxiety and trauma-related disorders. Pharmacol Ther 149:150-190.
2. Maren S & Holmes A (2016) Stress and fear extinction. Neuropsychopharmacology 41 (1):58.
3. Parsons RG & Ressler KJ (2013) Implications of memory modulation for post-traumatic stress and fear disorders. Nature neuroscience 16(2):146.
4. Herry C, et al. (2008) Switching on and off fear by distinct neuronal circuits. Nature 454(7204):600-606.
5. Jasnow AM, et al. (2013) Thy1-expressing neurons in the basolateral amygdala may mediate fear inhibition. Journal of Neuroscience 33(25):10396-10404.
6. McCullough KM, et al. (2016) Molecular characterization of Thy1 expressing fear-inhibiting neurons within the basolateral amygdala. Nature communications 7:13149.
By Joshua Au Yeung- Academic Clinical Fellow in Clinical Pharmacology and therapeutics in Guys St Thomas Hospital NHS Trust
In a culture of miracle cures and quick-fixes, we are constantly bombarded with sensationalist headlines of fad diets that promise instant weight loss or nonsensical health improvements. Having to weave through headlines, blogs, and celebrity endorsements of the new cancer cure or super-drug can make it difficult to know whether any of these claims bear any real weight or evidence-base.
A diet that has a growing body of evidence of health benefits is a high nitrate diet. Nitrate (NO3-) is a molecule consisting of one central nitrogen atom surrounded by three identically bonded oxygen atoms. Interestingly, nitrate has a long history as an explosive agent, being a major component of gunpowder. Nitrates are also common constituents of our diets found in abundance in fruits and vegetables, particularly in beetroot and green leafy vegetables. Dietary nitrate increases the systemic availability of nitric oxide, which in turn regulates several physiological processes that improve cardiac blood flow and exercise tolerance such as arterial vasodilation, blood pressure lowering, decreasing arterial wall stiffness and improving perfusion to muscles [1].
Evidence of beneficial effects of beetroot juice, which contains high amounts of nitrate, was first demonstrated in a study by Webb et al. that showed beetroot juice can reduce blood pressure by 10/8 mmHg (a normal blood pressure being 120/80 mmHg) and improve blood flow to the forearm [2]. A later systematic review showed that beetroot juice supplementation improved efficiency and performance in various time trials for endurance athletes in cycling and marathon runners [3]. Similarly, The Dietary Approaches to Stop Hypertension (DASH) study showed that a ‘combination diet’ high in fruit, green leafy vegetables and low?fat dairy produce reduced blood pressure and decrease risk of ischaemic stroke and coronary artery disease compared with a control diet [4].
The beneficial vascular effects of dietary nitrate in preventing and treating neuro-vascular disease are currently being explored. Stroke and dementia have a large mortality and disability-burden on United Kingdom’s aging population. In 2017 Bondonno et al. found that higher vegetable nitrate was associated with a decreased carotid artery wall thickness and a 17% lower risk of stroke [5]. Another study showed that a higher nitrate diet led to increased regional cerebral perfusion in frontal lobe white matter; critical areas involved in executive functioning. This may be a potential target to stave off the onset of dementia [6].
Fad diets come in and out of fashion on a weekly basis. So far, evidence suggests that eating beetroots, or a diet high in nitrate content, can reduce your cardiovascular risk and improve exercise tolerance. However, further high quality research needs to be conducted regarding whether beetroot is a viable treatment for stroke or dementia prevention. Until then, at the risk of sounding sensationalist, there’s no harm in grabbing a beetroot juice the next time you’re out brunching with your friends.
References
1. Khatri J, Mills CE, Maskell P, Odongerel C, Webb AJ. It is rocket science – why dietary nitrate is hard to ‘beet’! Part I: twists and turns in the realization of the nitrate– nitrite–NO pathway. (2017) Vol. 83, British Journal of Clinical Pharmacology. Blackwell Publishing Ltd; pp. 129–39.
2. Webb AJ, et al. (2008) Acute blood pressure lowering, vasoprotective, and antiplatelet properties of dietary nitrate via bioconversion to nitrite. Hypertension 51(3):784–90.
3. Domínguez R, et al. (2017) Effects of beetroot juice supplementation on cardiorespiratory endurance in athletes. A systematic review. Vol. 9, Nutrients MDPI AG.
4. Appel LJ, et al. (1997) A Clinical Trial of the Effects of Dietary Patterns on Blood Pressure. N Engl J Med [Internet]. 1997 Apr 17 [cited 2020 Jan 8];336(16):1117–24. Available from: http://www.nejm.org/doi/abs/10.1056/NEJM199704173361601
5. Bondonno CP, et al. (2017) Association of Vegetable Nitrate Intake with Carotid Atherosclerosis and Ischemic Cerebrovascular Disease in Older Women. Stroke 48(7):1724–9.
6. Presley TD, et al. (2011) Acute effect of a high nitrate diet on brain perfusion in older adults. Nitric Oxide - Biol Chem 24(1):34–42.
Editor-In-Chief:
Jayanthiny Kangatharan, PhD
Editors:
MIrza Ali Redah, Francesco Berti, PhD, Sebastian Birtles, Jack Cooper, Nerissa Culi, Daniel Dabbs, Matthew Herbert, Jayanthiny Kangatharan, PhD, Andreea Pantiru, Laura Riggall, Ryan Stanyard, Marco Travaglio
By Laura Riggall, PhD student in Neuroscience, University College London
Parkinson’s disease (PD) is the second most common neurodegenerative disorder characterised by motor, cognitive, psychiatric, and autonomic dysfunction [1]. This is due to a progressive loss of dopaminergic neurons in the substantia nigra, associated with the formation of fibrillar aggregates of α-synuclein and other proteins. Although 90% of cases are idiopathic, missense mutations in the gene encoding leucine-rich repeat kinase 2 (LRRK2) are the most common cause of autosomal dominant PD, of which the G2019S mutation is the most prevalent [2].
Such mutations may confer a toxic gain-of-function, leading to an increase in LRRK2 kinase activity [3] that subsequently affects mechanisms that maintain endoplasmic reticulum, vesicle trafficking, and autophagy [4-5]. As a result, LRRK2 inhibition represents a genetically-validated approach to treat PD patients.
Interestingly, the prevalence and penetrance of LRRK2 mutations varies between populations. A recent study determined that A419V, R1628P, and G2385R mutations increased the risk of PD in East Asian populations, whereas R1398H had the opposite effect. G2019S increased PD risk in European/West Asian and mixed populations, while R1441C/G/H increased risk in European/West Asian populations only [6]. The distribution of LRRK2 mutations therefore has implications for the treatment of PD on a global scale.
Various approaches are currently underway to establish treatments for PD. One of the most exciting is the collaboration between GlaxoSmithKline and consumer genetics and research company 23andMe. Over 5 million customers have signed up to 23andMe. The company’s state-of-the-art genotyping technologies measure more than 600,000 variants in each genome. By identifying those with LRRK2 variants, this programme has the potential to rapidly realise effective clinical proof-of-concept and aid trial recruitment [7-9].
The Michael J. Fox Foundation has also been spearheading efforts towards developing promising inhibitors by, for example, devising the LRRK2 Safety Initiative. Although pulmonary toxicity once threatened the development of this drug class, critical safety data provided by the initiative showed that these changes were reversible and not associated with functional impairment. This allowed the development of LRRK2 inhibitors from Genentech (that have since been sold to Denali Therapeutics), Merck and Pfizer to move forward [10]. Denali Therapeutics is now leading the way, recently reporting successful Phase I data [11].
It has been shown that pipeline drug targets with human genetic evidence of disease association are twice as likely to lead to approved drugs [12-13]. There is therefore vast potential for human genetics to transform the future of healthcare. Well conducted studies that identify clearly causal genes may hold the key to realising the next generation of therapies.
References:
1. Kalia, L.V. & Lang, A.E. (2016). Parkinson disease in 2015: Evolving basic, pathological and clinical concepts in PD. Nat Rev Neurol., 12: 65-66.
2. Zimprich, A., et al. (2004). Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron, 44: 601-607.
3. Greggio, E., et al. (2006). Kinase activity is required for the toxic effects of mutant LRRK2/dardarin. Neurobiol Dis., 23: 329-341.
4. Steger, M., et al. (2016). Phosphoproteomics reveals that Parkinson’s disease kinase LRRK2 regulates a subset of Rab GTPases. ELife, 5: e12813.
5. Di Maio, R., et al. (2018). LRRK2 activation in idiopathic Parkinson’s disease. Sci Transl Med., 10 (451): eaar5429
6. Shu, L., et al. (2019). A comprehensive analysis of population differences in LRRK2 variant distribution in Parkinson’s disease. Front Aging Neurosci., 11: 13.
7. GlaxoSmithKline plc. [online], GSK and 23andMe sign agreement to leverage genetic insights for the development of novel medicines. Available at: https://www.gsk.com/en-gb/media/press-releases/gsk-and-23andme-sign-agreement-to-leverage-genetic-insights-for-the-development-of-novel-medicines/ [Accessed 11th July 2019].
8. Wojcicki, A., 23andMeBlog [online], A note on 23andMe’s New Collaboration with GSK. Available at: https://blog.23andme.com/news/a-note-on-23andmes-new-collaboration-with-gsk/ [Accessed 29th July 2019].
9. George, S., Guardian News and Media Limited [online], Will genetics unlock a new treatment for Parkinson’s disease?. Available at: https://www.theguardian.com/breakthrough-science/2019/feb/28/will-genetics-unlock-a-new-treatment-for-parkinsons [Accessed 12th July 2019].
10.The Michael J. Fox Foundation [online], LRRK2 Safety Initiative. Available at: https://www.michaeljfox.org/lrrk2-safety-initiative [Accessed 12th August 2019].
11. Denali Therapeutics Inc. [online], Denali Therapeutics Announces Positive Clinical Results From LRRK2 Inhibitor Program For Parkinson’s Disease. Available at: http://investors.denalitherapeutics.com/news-releases/news-release-details/denali-therapeutics-announces-positive-clinical-results-lrrk2#ir-pages [Accessed 10th July 2019].
12. King, E. A., et al. (2019). Are drug targets with genetic support twice as likely to be approved? Revised estimates of the impact of genetic support for drug mechanisms on the probability of drug approval. bioRxiv., preprint.
13. Nelson, M. R., et al. (2015). The support of human genetic evidence for approved drug indications. Nat Genet., 47(8): 856-860.
By Shanice Bailey, PhD, Early Career Researcher, University of Cambridge
In 1992, John Gray’s book ‘Men are from Mars, Women are from Venus’, fuelled public debate about whether male and female brains are “wired” differently. The topic has continued to garner public attention due to huge societal implications on gender identification and cognitive ability. Nevertheless, empirical evidence for such differences have not been demonstrated in humans. Comparatively, decades of scientific research in model organisms such as worms, fruit flies and rodents tell a very clear story. Male and female brains of these species exhibit sexual dimorphism¹ ², and studies have largely focused on the circuitry underlying social behaviours such as courtship, mating, parenting and aggression. But why are sexually dimorphic circuits of interest in these models?
Animals exhibit sexually dimorphic behaviour to the same sensory signals which make sex-determined differences in the brain very intriguing. For example, detection of pup pheromones promote maternal behaviour in a female mouse, but trigger aggression in virgin males. Many of these behaviours are instinctive and therefore their underlying circuits considered to be genetically predetermined, making innate behaviours particularly powerful for the study of the neural basis of behaviour. Consequently sexual dimorphism can be leveraged to examine the different strategies implemented by nervous systems to tackle a plethora of sensory inputs and produce an appropriate motor output.
Researchers at the University of California investigate the functional relationship between sex-based differences in neural circuits and the genetic profiles of each cellular component, in the mouse medial amygdala (MeA) during pup-directed interactions. The MeA, which encodes ethologically relevant cues in a sexually dimorphic manner³, receives olfactory inputs and relays this information to the hypothalamus, a node in the brain associated with social behaviours. Using optogenetics, Chen et al found that high intensity activation of MeA GABAergic neurons upregulated the expression of pup grooming in females?. In contrast, this manipulation promoted infanticidal behaviour in males, although interestingly, behavioural responses were starkly amenable to the intensity of light stimulation. Low intensity activation enhanced the display of grooming behaviours, whereas females displayed grooming towards pups regardless of the degree of light intensity. Complementary single-cell transcriptomics identified sexually dimorphic gene expression within the GABAergic neuronal population relative to glutamatergic neurons within the MeA, but no other variance in cell-type identity or number were observed. The authors posit that functional differences in MeA responses between sexes can be ascribed to these transcriptional distinctions, presumably mediated by hormones. Such studies expand our understanding of sexual dimorphism in the mammalian brain from the level of molecules to circuits for the generation of divergent behaviour.
References:
By Grace Poole, Medical student, St George's University of London
850,000 individuals are living with dementia within the UK. A figure predicted to increase to one million by 2025, making it the sixth leading cause of death worldwide. Alzheimer’s disease (AD) is the most common form of dementia, comprising 60-80% of all cases [1].
AD is clinically diagnosed when patients present with marked cognitive impairment that significantly impacts their quality of life [2]. Impairment may present as deficits in attention, working memory and executive function. Yet, by the time cognitive impairment manifests, extensive neuronal loss has already occurred. It is estimated that 60% of neurons in layer II of the entorhinal cortex, a region essential for spatial navigation, have degenerated by this stage [3]. As a result, there is immense pressure to identify individuals with AD earlier. In fact, it is hypothesised that earlier treatment may hault the progression of disease and lead to better clinical outcomes.
Mild cognitive impairment (MCI) is a prodromal stage of AD. Whilst people with MCI are more likely to develop AD, not all individuals will progress [4]. Biochemical biomarkers such as Amyloid-b 42 (Ab42), phosphorylated tau (pTau) and total tau (Tau) in the cerebrospinal fluid (CSF) help predict MCI progression to AD [5]. However, to obtain CSF, patients must withstand a lumbar puncture. This carries significant risks of infection, brain herniation and pain [6]. Thus, biochemical biomarkers are not recommended for screening and non-invasive diagnostic procedures would be preferred.
An exciting collaboration between the University of Cambridge and University College London aims to address this problem by developing a non-invasive clinical biomarker. Their study used virtual reality (VR) technology with a paradigm testing for entorhinal cortex dysfunction. The aim was to determine whether this could identify individuals with MCI at a greater risk of developing AD [7].
From each patient with MCI, a sample of CSF was taken to assess for the aforementioned biomarkers. Subsequently, participants used VR to walk to three different locations. Upon reaching the final destination, they were asked to return to their remembered original location. MCI subjects with CSF biomarkers performed worse on this task compared to those without. Interestingly, this novel paradigm was better at identifying high-risk individuals than the Addenbrooks Cognitive Examination (ACE-III), a test commonly utilised within memory clinics [7,8]. This evidence shows that VR may represent a new sensitive and specific tool for early diagnosis. In addition, VR is non-invasive, provides a safe environment and can be reproduced across multiple clinics.
There are, however, limitations that restrict the use of this novel biomarker within a clinical setting. Currently, all licensed treatments for AD are symptomatic, meaning there is limited benefit of diagnosing AD earlier until disease modifying treatments exist. If treatments delaying the progression of MCI to AD are developed, VR is poised to be an effective tool for early diagnosis.
Bibliography
1. Prince M et al (2013) The global prevalence of dementia: a systematic review and meta-analysis. Alzheimer’s & Dementia 9(1): 63–75.
2. Alzheimer’s Society (2019) The progression of Alzheimer’s disease. Available at: https://www.alzheimers.org.uk/about-dementia/symptoms-and-diagnosis/how-dementia-progresses/progression-alzheimers-disease [Accessed: 6th July 2019]
3. Braak H., & Del Tredici K. (2015) The preclinical phase of the pathological process underlying sporadic Alzheimer’s disease. Brain 138(10), 2814–2833.
4. Alzheimer’s Society (2019). Mild Cognitive Impairment. Available at https://www.alzheimers.org.uk/about-dementia/types-dementia/mild-cognitive-impairment-mci [Accessed: 6th July 2019]
5. Blennow K & Zetterberg H (2018) Biomarkers for Alzheimer’s disease: current status and prospects for the future. Journal of Internal Medicine 284(6):643-663
6. Patient plus (2015) Lumbar Puncture. Available at: https://patient.info/doctor/lumbar-puncture-pro#nav-4 [Accessed: 6th July 2019]
7. Howett D et al. (2019) Differentiation of mild cognitive impairment using an entorhinal cortex-based test of virtual reality navigation. Brain 142(6):1751-1766
8. Alzheimer’s society (2015) Helping you to assess cognition. [Accessed: 6th July 2019]
By Page Stevenson, Undergraduate student in Neuroscience, University of Leeds
Our population is rapidly ageing and as a result, the number of people diagnosed with dementia is expected to increase by 204% from 2018 to 2050 (1). Neurodegenerative diseases, such as Alzheimer’s and Parkinson’s, are one of the primary causes of dementia. Excessive immune responses play a role in the progression of these diseases (2). It is vital we identify effective treatments to minimise the suffering of many.
One area of research into neurodegeneration focuses on the role of microglia, immune cells found in the nervous system, in the exaggerated host defense.
In healthy brains, microglia mediate the immune response in the brain. Normally, microglia are in a quiescent non-activated state. When activated by damage/infection, they produce cytokines and other molecules in a pro-inflammatory response (3). There is also an anti-inflammatory microglial state, which resolves the inflammation. However, microglia can become chronically activated: this is where problems arise. When pro-inflammatory cytokines are produced in excess, this exaggerated immune response will lead to responses including increased chemotaxis of proteins which activate leukocytes. This can destroy cells unnecessarily and contribute to neurodegeneration (4).
Drugs that can reduce the microglial pro-inflammatory state or promote the anti-inflammatory state could be used therapeutically to treat neurodegenerative diseases. One such class of molecules are histone deacetylase (HDAC) inhibitors.
Histones are proteins associated with DNA to package it into a compact structure called a nucleosome. DNA wrapped around histones is more difficult to transcribe. HDACs remove acetyl groups from histones; this deacetylation promotes compact structure so reduces gene expression (5). Besides histones, acetylated proteins are also believed to be responsible for anti-inflammatory responses (6). Inhibiting HDACs using HDAC inhibitors could therefore promote anti-inflammation.
To determine a microglial response, the expression levels of specific cytokines associated with pro-inflammatory states (such as IL-6) and anti-inflammatory states (such as IL-10) are measured. Treatment with a stimulant known to evoke a microglial inflammatory response caused an increase in the production of IL-6 (7). However, this production was inhibited when the cells were also incubated with a number of HDAC inhibitors (8). This indicates that the microglial phenotype has been shifted towards the resting state.
That said, we cannot simply administer HDAC inhibitors to patients and expect a drastic improvement. HDACs are widely expressed and play roles in many physiological processes. HDAC inhibitors which could selectively target HDACs involved in the inflammatory process would therefore provide greater therapeutic benefit. This applies not only to neurodegeneration but to other pathologies such as sport injuries where inflammation is involved.
References:
1. World Health Organisation (2019) Dementia.[Online]. [Accessed 9 July 2019]. Available from: https://www.who.int/news-room/fact-sheets/detail/dementia
2. Labzin, L.I., Heneka, M.T. and Latz, E. (2018) Innate Immunity and Neurodegeneration. Annual Review of Medicine. 29(69). pp. 437-49
3. Block, M.L. and Hong, J.S. (2007) Chronic microglial activation and progressive dopaminergic neurotoxicity. Biochemical Society Transactions. 35(5). pp.1127-32
4. Zhang, J. and Jianxiong, A. (2009) Cytokines, Inflammation and Pain. International Anesthesiology Clinics. 45(2). pp. 27-37
5. Annunziato, A.T. and Hansen, J. C. (2000) Role of Histone Acetylation in the Assembly and Modulation of Chromatin Structures. Gene Expression. 9(1-2). pp.37-61.
6. Durham, B.S., Grigg, R. and Wood, I.C. (2017) Inhibition of histone deacetylase 1 or 2 reduces induced cytokine expression in microglia through a protein synthesis independent mechanism. Journal of Neurochemistry.143. pp.214-224.
7. Minogue, A.M., Barrett, J.P. and Lynch, M.A. (2012) LPS-induced release of IL-6 from glia modulates production of IL-1β in a JAK2-dependent manner. Journal of Neuroinflammation. 9. 126.
8. Kannan, V. et al. (2013) Histone deacetylase inhibitors suppress immune activation in primary mouse microglia. Journal of Neuroscience Research. 91(9). pp.1133-42.
By Beverley Darkin, MSc student in Applied Neuroscience, King's College London
Some individuals are less susceptible to mental health disorders than others. The reasons for this are unclear. One approach to understanding such resilience is to investigate a specific disorder, such as post-traumatic stress disorder (PTSD).
PTSD is an anxiety disorder caused by exposure to frightening or life threating situations. Symptoms include nightmares and flashbacks, hypervigilance, avoidance, and altered cognition and mood [1]. A substantial literature on PTSD has developed over the past decade, seeking to understand the molecular basis of the condition [2]. Most of these studies are epigenetic or candidate gene analyses. They involve selecting genes to investigate on the basis of biological hypotheses, targeting polymorphisms in or around the gene of interest. PTSD is characterised by fear and stress, and candidate gene studies focus on associated biological systems such as the body’s stress response system, the hypothalamic-pituitary-adrenal axis [3].
Genome wide association, by contrast, is a genomic technique that searches for single-nucleotide polymorphisms (SNPs; substitutions in single nucleotides) across the genome. There is no attempt to predict which genes might be involved. Rather, SNPs found to be more frequent in PTSD sufferers can be associated with the disorder. Being hypothesis free, GWAS can reveal novel genetic variants not considered under the traditional approach. In 2017 there were just eight published GWAS for PTSD [4] but cheap chip-based genotyping, which allows thousands of SNPs to be assessed, is increasing their popularity.
GWAS are not without challenges. PTSD is a polygenic disorder, meaning numerous genes each contribute a small increase in risk. A further problem is that PTSD studies are not consistent in the way they define cases or controls [5]. To overcome these challenges, the Psychiatric Genomics Consortium for PTSD – a large-scale international collaboration of over 200 scientists – was set up to combine expertise and pool data. These scientists hope collaboration will substantially increase sample sizes, providing the statistical power needed to uncover the genes that put individuals at risk. In 2018 the sample included more than 32,000 PTSD cases and 100,000 trauma-exposed controls. New data is being added all the time [6].
It is early days but, according to consortium co-chair Dr Caroline Nievergelt, initial findings show that SNP-based heritability is higher for women than in men. There is also significant shared genetic risk between PTSD and other psychiatric disorders. By seeking to understand the molecular basis of PTSD there is also the possibility of unravelling the neurobiological underpinnings of resilience. Given the shared genetic risk between PTSD and other disorders, the benefits could extend well beyond PTSD.
Acknowledgements: Thank you to Dr Patricia Zunszain and Dr Aoife Keohane, King’s College London, for their guidance and support.
References
By Elene Nicola, Graduate Student in Psychology and Neuroscience, University of Keele
Dietary patterns such as poor intake of vital food groups, lacking appetite, overwhelming desires for sugary foods and skipping meals are usually observed in people suffering from depression or anxiety disorders. It has been documented that many of these food patterns are usually present prior to the onset of these illnesses (1)(2). This highlights that nutrition and diet may be potential risk factors for the development of depression and anxiety disorders.
Research has shown that nutritional deficiencies can influence brain structure and function, importantly in the chemical communications between neurones in networks closely associated with mood, depression and anxiety (3). Successful chemical communications involve several steps, where the foundation of the process relies exclusively on the synthesis and regulated release of neurotransmitters (9). Disruption in this fundamental process including a deficiency in these neurotransmitters, may be a crucial mechanism underlying depression and other mental illnesses. This is observed in the monoamine hypothesis of depression and is well established as the target of action for many antidepressants, i.e. selective-serotonin-reuptake-inhibitors (SSRIs) (7). To clarify, the monoamine hypothesis of depression proposes that depressive disorders are due a to functional deficiency of monoamine neurotransmitters (serotonin, noradrenaline and to a lesser degree dopamine) and mania is caused by a functional excess (9).
Interestingly, there are several amino acids that cannot be made within the body itself and can only be supplied through diet and ingestion (4). These are known as essential amino acids. Tryptophan is one of these essential amino acids and is rich in foods such as nuts, cheese, eggs, chicken, seeds and oats (4). Tryptophan is a necessity for the production of serotonin, where a deficiency may explain the neurogenic processes seen in depression and other mental health illnesses (as observed in the monoamine hypothesis). Taken together, this suggests that the rate of serotonin being synthesised and released from neurons is dependent on tryptophan concentrations within the brain (5). Indeed, lower quantities have been shown to contribute to elevated levels of anxiety and depression (6).
This rate of regulation is extremely sensitive to relatively small physiological changes in tryptophan concentrations. Even slight variations in diet could theoretically have a large effect on the synthesis and release of serotonin and in turn modify and fluctuate levels of anxiousness and depressive states (8). As well as this, the flux of tryptophan into the brain is dependent on the type of foods consumed, where other similar amino acids such as tyrosine and phenylalanine compete for transport via the blood-brain barrier. Dietary carbohydrates raise the plasma tryptophan ratio in blood, thus facilitating tryptophan’s entry into the brain. In contrast, dietary protein lowers the plasma tryptophan ratio, as they contain higher levels of other amino acids compared to tryptophan (11). Therefore, maximising consumption of carbohydrates rich in tryptophan may have a larger effect on alleviating depressive and anxious states in contrast to protein consumption.
Clinical studies show that the consumption of a high tryptophan diet improved mood significantly and led to reductions in depressive symptoms and anxiety levels, compared to participants with low tryptophan diets (typically below the recommended daily intake for tryptophan at 4 mg/kg of body weight as set by the World Health Organization) (4) (10). This suggests that altering diets to increase tryptophan consumption could modify the rate of serotonin synthesis and release within the brain leading to adjustments in mood and psychopathology with regards to depressive and anxiety disorders. This data may also suggest that psychopathology may be linked to metabolic disorders. Interventions that aim to alleviate nutritional deficiencies, such as dietary supplements or adjustments in food intake, could therefore produce therapeutic effects in mental health.
References:
1. Cooper, J. (2000). Diagnostic and Statistical Manual of Mental Disorders (4th edn, text revision) (DSM–IV–TR) Washington, DC: American Psychiatric
Association 2000. British Journal Of Psychiatry, 179(1), 85-85..
2. Sánchez-Villegas, A, et al. (2011) Dietary Fat Intake and the Risk of Depression: The SUN Project. Plos ONE, 6(1), 162-68.
3. Sathyanarayana Rao T, et al. (2008) Understanding nutrition, depression and mental illnesses. Indian Journal Of Psychiatry, 50(2):77.
4. Soh N & Walter G (2011). Tryptophan and depression: can diet alone be the answer?. Acta Neuropsychiatrica, 23(01): 3-11.
5. Lindseth G, et al. (2016). The effects of dietary tryptophan on affective disorders. Archives of Psychiatry Nursing, 2(29): 102-107.
6. Robinson O, et al. (2010). Mood state moderates the role of serotonin in cognitive biases. Journal Of Psychopharmacology, 24(4): 573-583.
7. Meyers S, (2000). Use of neurotransmitter precursors for treatment of depression. Alternative Medicine Reviews, (5): 64-71.
8. Fernstrom J, (1981). Dietary Precursors and Brain Neurotransmitter Formation. Annual Review Of Medicine, 32(1): 413-425.
9. Brigitta B. (2002). Pathophysiology of depression and mechanisms of treatment. Dialogues in clinical neuroscience, 4(1), 7–20.
10. Kapalka, G. (2010). Substances Involved in Neurotransmission. Nutritional and Herbal Therapies for Children and Adolescents, pp.71-99.
11. Wurtman, R, et al. (2003). Effects of normal meals rich in carbohydrates or proteins on plasma tryptophan and tyrosine ratios. The American Journal of Clinical
Nutrition, 77(1), pp.128-132.
Editor-In-Chief:
Jayanthiny Kangatharan, PhD
Editors:
Sebastian Birtles, Jack Cooper, Matthew Herbert, Jayanthiny Kangatharan, PhD, Leanne Kapa, Wajiha Munir, Oriol Pavón Arocas, Marco Travaglio, Fran van Heusden
By Carolyn Saund, PhD student in Research Methods in Psychological Sciences, University of Glasgow
Human-Computer interaction is hard, but our technological interfaces have improved over time. From terminal commands to touchpads, and text to voice interfaces, consumer electronics industries understand that ease-of-use is of the utmost importance to customers. The ultimate evolution of this idea is a frictionless Brain-Computer Interface (BCI).
These interfaces envisage a seductively convenient future: Machines that turn ideas to fully formed papers, gadgets that download memories so you never forget memories so you never forget, implanted devices for a wide array of medical conditions. With such tremendous possibility to break into the tech marketplace, a number of companies are working to make this dream a reality, and they are getting lots of money for it.
While French gaming company NextMind tries to let users control video games with their $5M disclosed funding, medical device companies like Kernel and MindMaze have raised upwards of $100M to realize dreams of memory extension and neurorehabilitation through Neural Interfaces. Californian NeuroPace has gotten over $200M for their implant that responds to neurological activity. And, despite being intensely secretive, NeuraLink has raised over $27M for claiming to one day provide “super-human cognition.”
These companies make assertions about their products based on sound research. Scientists showed humans are able to control interfaces on screens [7] and even prosthetics [5] using only their mind as early as 1991. Neurostimulation has shown to have profound effects on the ability to perceive empathy [4] and boost cognition [2]. We have even visualized memories based solely on brain activity [6]. This shows significant scientific progress in the field of BCI, but is that enough for these companies to succeed?
Going from a Proof-of-Concept to a product is a perilous journey. Looking at the field of social robotics, there are troubling comparisons, as demonstrated by the rise and fall of Jibo, Aido, Miro, Anki, and all your other two-syllable-end-vowel robotics companies. These companies were based on research from MIT, Oxford, and Harvard that said social robots are socially desirable [3] and medically helpful [1]. They managed to raise staggering amounts of money, often in the realm of $100M or more. They were run by charismatic, successful serial-entrepreneurs. But ultimately, these robotics companies were built on future bets founded on the premise that we have technologies which we just do not yet have.
The magic of BCI, like that of robots, captures the popular imagination. It’s important that someone dreams big and looks to the future -- pulls the popular zeitgeist towards the presently-impossible but theoretically-plausible. Going from unimaginable to imagined is no small feat, and these companies are filling and important role in that regard. But, as usual when non-experts are confronted with dramatic future visions, things are blown out of proportion too quickly. While BCIs show intriguing potential to transform future interactions with technology, until any company successfully brings a BCI product to market that consumers can purchase off-the-shelf, assertions of their immediate impact should be met with scepticism. This article in full together with references can be found at: https://www.bna.org.uk/publications/ bright-brains/bb-online/.
References
1. Broekens, J., Heerink, M., & Rosendal, H. (2009). Assistive social robots in elderly care: a review. Gerontechnology, 8(2), 94-103.
2. Enriquez-Geppert, S., Huster, R. J., & Herrmann, C. S. (2013). Boosting brain functions: Improving executive functions with behavioral training, neurostimulation, and neurofeedback. International journal of psychophysiology, 88(1), 1-16.
3. Fong, T., Nourbakhsh, I., & Dautenhahn, K. (2003). A survey of socially interactive robots. Robotics and autonomous systems, 42(3-4), 143-166.
4. Hétu, S., Taschereau-Dumouchel, V., & Jackson, P. L. (2012). Stimulating the brain to study social interactions and empathy. Brain Stimulation, 5(2), 95-102.
5. Muller-Putz, G. R., & Pfurtscheller, G. (2008). Control of an electrical prosthesis with an SSVEP-based BCI. IEEE Transactions on Biomedical Engineering, 55(1), 361-364.
6. Wagner, A. D., Schacter, D. L., Rotte, M., Koutstaal, W., Maril, A., Dale, A. M., ... & Buckner, R. L. (1998). Building memories: remembering and forgetting of verbal experiences as predicted by brain activity. Science, 281(5380), 1188-1191.
7. Wolpaw, J. R., McFarland, D. J., Neat, G. W., & Forneris, C. A. (1991). An EEG-based brain-computer interface for cursor control. Electroencephalography and clinical neurophysiology, 78(3), 252-259.
By Bo Yao, Lecturer in Cognitive Neuroscience, University of Manchester
When we check our Twitter feeds or read a novel before bed, we often “hear” people speak off the page. Although silent reading is soundless from an outsider’s perspective, it is in fact accompanied by a vivid speech experience inside the brain. Such inwardly audible, speech-like experience is called inner speech. It occupies at least 25% of our waking life and is pervasive in human cognition.
On the one hand, it plays important roles in working memory, language processing and behavior regulation. On the other, its dysfunction may lead to serious mental disorders such as rumination and auditory verbal hallucinations [1].
Despite the importance of inner speech in human cognition, its neural and cognitive underpinnings remain elusive. As a silent mental activity, inner speech used to be difficult to observe or measure objectively. Fortunately, neuroscientists have now developed modern techniques to ‘listen in’ to the brain’s private conversations in real time.
One useful technique is called functional magnetic resonance imaging (fMRI), which takes snapshots of the brain’s oxygen levels whilst it engages in different mental activities. Scientists found that parts of the brain’s auditory cortex, where actual speech is processed, are activated (i.e. bringing in more blood oxygen) during silent reading of particularly speech quotations (e.g. She rolled her eyes and said, “Brexit is a total nightmare!”) [2]. To decode and comprehend printed symbols, silent reading should only engage parts of the brain for visual and semantic processing. Since the auditory cortex is also activated by written words, it seems, after all that the brain can entertain itself by mentally filling in the auditory details, e.g. protagonists’ voices during reading.
Using intracranial electrodes in epileptic patients [3], scientists detected inner speech-related neural activity primarily in the ventral occipital temporal cortex where written symbols are mapped to the corresponding sounds, and later in the auditory cortex where these sounds are combined into a coherent experience of inner speech. Moreover, we seem more consciously aware of our inner speech when it coincides with the rhythms of neuronal excitability in the auditory cortex, as recently recorded using electroencephalograms (EEGs) from the scalps of healthy readers in our lab.
It has become clear that inner speech is associated with neural activity in the auditory cortex. Yet there is still a long way to go before we start to grasp the neural mechanism of inner speech processing. Can we decode one’s inner speech from their EEG recordings? What are the similarities and differences between inner speech and auditory verbal hallucinations in psychosis? What is certain, however, is that advances in technology will allow us to develop new methods and design better experiments to unveil the secrets of that little voice hidden within the skull.
References
1. Alderson-Day, B., & Fernyhough, C. (2015). Inner speech: development, cognitive functions, phenomenology, and neurobiology. Psychological bulletin, 141(5), 931.
2. Yao B, et al. (2011). Silent reading of direct versus indirect speech activates voice -selective areas in the auditory cortex. Journal of Cognitive Neuroscience 23(10):3146¬3152.
3. Perrone-Bertolotti M, et al. (2012). How silent is silent reading? Intracerebral evidence for top-down activation of temporal voice areas during reading. Journal of Neuroscience 32(49):17554–17562.
By Laura Riggall, PhD student in Neuroscience, University College London
Why are there no effective treatments for neurodegenerative diseases such as Alzheimer’s (AD) and Parkinson’s (PD)? Despite decades of research, their complexity has hindered therapeutic development. The application of preclinical imaging, including positron emission tomography (PET) and magnetic resonance imaging (MRI) in animal models, aims to change this.
Animal models that accurately represent patient-like phenotypes and pathologies are essential. They include those that are genetically engineered [1], and in combination with imaging, are powerful tools for providing early and highly predictive data for drug discovery and compound development. Preclinical imaging can also assess anatomical, physiological, and molecular and cellular processes [2], tracking disease progression over time non-invasively.
So far, imaging has allowed us to make great progress in the fight against neurodegenerative disease. For example, structural MRI is currently used to identify and monitor atrophy of the striatum, one of the most sensitive biomarkers available for Huntington’s disease (HD) [3,4].
Imaging is also providing new insights. By imaging the inflammatory response of microglia to Abeta plaques (a hallmark of AD) with PET via translocator protein in an AD mouse model, researchers were able to better predict disease progression than with the more traditional Abeta PET. AD mice with the greatest PET signal also showed better cognitive performance, suggesting that modifying a patient's microglial state may ameliorate cognitive decline [5].
Another study has shown that an amyloid-targeted molecular MRI probe called W20-SPIONs (a nanoparticle-antibody conjugate) bound to brain regions with amyloidogenic proteins, emitting a signal that distinguished PD and HD mice from controls. This indicates that W20-SPIONs have potential in early-stage diagnoses of these diseases, and represents a new strategy for testing the effectiveness of new treatments for neurodegeneration [6].
Preclinical imaging also enables researchers to combine traditionally separate modalities to obtain anatomical and functional parameters simultaneously. For example, functional optical coherence tomography and angiography are being combined in AD mouse models to determine whether neurodegeneration is preceded by a weakened response of blood vessels to neural impulses from individual photoreceptors in the retina [7].
Overall, preclinical imaging has great potential in the drug discovery and development process for neurodegenerative disease. Technological advancements, not just in modalities but also in data analysis and software development, are ever-occurring, enabling preclinical imaging to grow exponentially. Increasingly sophisticated and well-validated technologies, probes, and biomarkers will ultimately enable imaging to accelerate the development of much-needed treatments for patients.
References
[1] Wendler, A. & Wehling, M. (2010). The translatability of animal models for clinical development: biomarkers and disease models. Curr Opin Pharmacol., 10 (5): 601-6.
[2] Cunha, L., et al. (2014). Preclinical imaging: an essential ally in modern biosciences. Mol Diagn Ther., 18 (2): 153-73.
[3] Tabrizi, S.J., et al. (2011). Biological and clinical changes in premanifest and early stage Huntington’s disease in the TRACK-HD study: the 12-month longitudinal analysis. Lancet Neurol., 10 (1): 31-42.
[4] Tabrizi S.J., et al. (2012) Potential endpoints for clinical trials in premanifest and early Huntington’s disease in the TRACK-HD study: analysis of 24 month observational data. Lancet Neurol., 11(1): 42–53.
[5] Focke, C., et al. (2019). Early and Longitudinal Microglial Activation but Not Amyloid Accumulation Predicts Cognitive Outcome in PS2APP Mice. J Nucl Med., 60 (4): 548-554.
[6] Liu, X.G., et al. (2019). ScFv-conjugated superparamagnetic iron oxide nanoparticles for MRI-based diagnosis in transgenic mouse models of Parkinson’s and Huntington’s diseases. Brain Res., 15;1707: 141-153.
[7] Parmet, S. (University of Illinois at Chicago), Detecting eye and brain disease earlier. UIC Today [online]. Available at: https://today.uic.edu/detecting-eye-and-brain-disease-earlier [Accessed 22nd April 2019].
By Charlott Repschlager, PhD student in Neuroscience, King's College London
Neurodegenerative disorders are usually associated with an elderly population and people tend to think of dementia and Alzheimer’s Disease. Some people might even think about neurodegeneration in younger adults, such as those with multiple sclerosis. But very few people think of children when they are told about neurodegeneration. Although rare, those disorders unfortunately exist, and my PhD work has been focusing on one group in particular, the neuronal ceroid lipofuscinoses (NCLs).
NCLs, also known as Batten Disease, are a group of rare, but fatal childhood illnesses. While their genetic background is highly diverse, all the mutations affect lysosomes, which is why NCLs are described as lysosomal disorders. Lysosomes are cell organelles important for waste disposal. They are a critical component of cellular metabolism and their dysfunction ultimately leads to cell death. The defective proteins that cause Batten Disease can be divided into two main groups: those that are soluble within the lysosome and those that are bound to a membrane. These proteins are used to name the individual forms of NCL, e.g. CLN2 disease means that there is a defect in the Cln2 gene and therefore in the Cln2 protein. My research focuses on transmembrane protein-deficient forms of NCL and in particular on CLN3 and CLN7 disease.
While NCLs are still regarded as incurable, research into possible treatment options has progressed significantly in recent years and the first drug to address the cause of an NCL (CLN2) has been approved in the US recently. The therapy directly administers the missing enzyme to the cells that need it to function properly. While this approach does not work in the forms of NCL in which the protein is bound to a membrane, all therapies have one thing in common; they work better when they are targeted. Quite recently studies have shown that administering Cln1 gene therapy to the brain and spinal cord of mice which lack Cln1 proteins has a better effect that injecting it into either site alone. This shows how important it is to not only know how to treat a disease, but also where.
Based on this research I am now investigating the spinal cords of mice with CLN3 and CLN7 disease to establish whether they are as involved in the disease as it is in CLN1 disease. This research will hopefully not only pave the way to a better understanding and treatment of disease progression in NCL, but also shed some light on other neurodegenerative disorders.
References
Cooper J (2010) The neuronal ceroid lipofuscinoses: the same, but different? Biochemical Society Transactions 38(6):1448-1452.
Cooper J, et al. (2015) Towards a new understanding of NCL pathogenesis. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease 1852(10, Part B):2256-2261.
Cooper J & Nelvagal H (2017) Progress toward Fulfilling the Potential of Immunomodulation in Childhood Neurodegeneration? Molecular Therapy 25(8):8-10.
Cotman S, et al. (2017) Future perspectives: Moving towards NCL treatments. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease 1852(10, Part B):2336-2338.
Coutinho MF & Alves, S (2016) From rare to common and back again: 60years of lysosomal dysfunction. Molecular genetics and metabolism 117(2):53-65.
Katz M, et al. (2015) AAV gene transfer delays disease onset in a TPP1-deficient canine model of the late infantile form of Batten disease. Science Translational Medicine 7(313):313ra180-313ra180.
Neverman N, et al. (2015) Experimental therapies in the neuronal ceroid lipofuscinoses. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease 1852(10):2292-2300.
Schulz A, et al. (2017) Long-term safety and efficacy of intracerebroventricular enzyme replacement therapy with cerliponase alfa in children with CLN2 disease: interim results from an ongoing multicenter, multinational extension study. Molecular Genetics and Metabolism 120(1):S120.
Shyng C,e t al. (2017) Synergistic effects of treating the spinal cord and brain in CLN1 disease. Proceedings of the National Academy of Sciences 114(29):E5920-E5929.
Warrier V, et al. (2013) Genetic basis and phenotypic correlations of the neuronal ceroid lipofusinoses. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease 1832(11):1827-1830.
By Caroline Casey, PhD student in Neuroscience, University College London
For over a decade now, induced pluripotent stem cells (iPSC) have been expanding the possibilities of neurological research. Adult cells, commonly taken from skin are ‘reprogrammed’ back to a stem cell state, enabling them to differentiate into any known cell type. This allows the study of previously out of reach areas, as well as the potential for individualised therapy. The human brain is no exception.
Huntington’s disease (HD) is a neurodegenerative disease in which a genetic mutation causes a toxic protein to accumulate, wreaking havoc in the brain and peripheral nervous system. Certain brain regions are vulnerable to toxicity, including neurons of the striatum and layers of the cortex. These two regions are connected by axonal projections which transmit information, defined as the corticostriatal (CS) pathway. Researchers have established from animal models that the breakdown of this pathway in HD underlies multiple symptoms.
This pathway has been the focus of my PhD studies, which have involved generating a cohort of iPSC-derived medium spiny neurons (MSN) and cortical neurons, from cells donated by a HD family, in which three children developed the disease.
By comparing these HD neurons with those differentiated from their maternal control, it was possible to observe the emergence of HD phenotypes in both MSNs and cortical neurons.
When MSNs were exposed to stress, such as the removal of key growth factors, the HD neurons exhibited an elevated level of cell death, suggesting they are more vulnerable to stress-induced toxicity. Both cell types also exhibited differences in their level of adhesion to culture substrates; HD neurons detached more easily than control cultures, possibly suggesting a deficiency in integrin binding. Finally, cortical neurons exhibited drastic differences in their morphology and axonal projection. Interestingly, it could be argued that if cortical projection is deficient in HD, the CS pathway may not develop normally.
To test this, and to identify if it was possible to generate a human brain pathway in the lab, a series of co-culture experiments were completed. This entailed the design and optimisation of specialised culture dishes called microfluidic chambers. These chambers physically separate the cell bodies of MSNs and cortical neurons, whilst still allowing axonal projection and connectivity to be established, thus the CS pathway was created in a dish. Preliminary results suggest that both control and HD neurons are able to establish connections with each other. This work is evidence that we can now study human brain pathways implicated in disease prior to post-mortem, and may be able to unveil early stage pathology.
Please take a look at the following links to recommended review articles for further information on this research topic.
https://onlinelibrary.wiley.com/doi/full/10.1111/cns.12828
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5181679/
https://www.frontiersin.org/articles/10.3389/fnhum.2016.00317/full
https://onlinelibrary.wiley.com/doi/full/10.1111/cns.12828
By Josh Au-Yeung, Stroke Registrar, NHS, Northern Care Alliance
In 1965, Gordon E. Moore—the co-founder of Intel postulated that the number of transistors that can be packed into a given unit of space will double every two years. This postulation predicted that computers would become exponentially more powerful and more affordable as time went by. Moore’s law still holds true today and is applicable to most technology; computers and smartphones have advanced at an alarming rate with increasing accessibility. It wasn’t long ago that virtual reality (VR), digital automation and artificial intelligence sounded like a distant dream from a sci-fi novel. Now, these processes have gradually and surreptitiously become ingrained as a vital and integral part of our existence.
One exciting development is the use of VR to improve neurorehabilitation outcomes for patients. VR based rehabilitation is an umbrella term for novel and potentially useful technologies that allow users to interact in 3 dimensions with a computer-generated virtual world. This can range from a simple computer screen to a sophisticated robotic device.
Stroke is a leading cause of adult disability in the world. In typical stroke patients, an infarction has disrupted the brain’s blood supply to the point where irreversible damage has occurred. In the acute phase of stroke, neuronal connections are severed and the patient can suffer detrimental deficits in their motor, sensory, balance, co-ordination and higher cognitive function. In the majority of stroke patients aspirin and physical therapy are still the mainstay of treatment, with a focus on rewiring lost neuronal connections, developing new compensating areas through neuroplasticity and motor learning, and neuromuscular co-ordination.
Rehabilitation physiotherapy is often tediously repetitive and require constant supervision. The advantages of VR therapy over traditional therapy approaches is that they can give people an opportunity to practise everyday activities that are not or cannot be practised within the hospital environment. Furthermore, there are several features of VR programs that might mean that patients spend more time in therapy: for example, the activity might be more engaging or motivating, the therapy is easily accessible and may require less staff supervision.
The gaming industry has developed a variety of VR systems for home use, making this technology both affordable and accessible with potential application in community settings. In particular, these technologies allow for increasing rehabilitation intensity required for induction of neuroplasticity.
Although the literature is limited, there are many promising outcomes from using VR as an alternative or adjunct to conventional physical therapy. VR rehabilitation programmes have recently received FDA approval in the US. Indeed, a systematic review found that the use of VR was found to be significantly more effective than conventional therapy in improving upper limb function and improving activities of daily living function (1).
We are amongst a new age of automation and technology. As VR continues to advance within healthcare and specifically within neurological space, it is important new technologies are designed with thought and care and targeted at specific diseases. Our approach to new-found technologies and the ethics that underpin its usage will shape the future of our civilisation, but that is a conversation for another day.
References
(1) Laver K, et al. (2015) Virtual reality for stroke rehabilitation: an abridged version of a Cochrane review. Eur J Phys Rehabil Med. 51(4):497-506
By Yu Yizhou, Undergraduate Student in Biological Sciences, Imperial College London
A recent publication in Nature elucidated the neural mechanisms that enable the consolidation of different type of memory during sleep, proposing that the activation of a hippocampus-dependent mechanism during sleep mediates the formation of hippocampus-independent long-term memory in mice (Figure 1). This work advances our understanding of the role of the hippocampus in memory consolidation.
Background
Sleep is common to most animals, but its exact function remains unclear. Several theories have been proposed to elucidate its relationship with memory consolidation [1-3], but the specific mechanisms remain elusive. For instance, recent memories can be replayed during sleep. In fact, the neuronal activity patterns (recorded via electroencephalography, EEG) during a learning session (encoding) resemble those recorded during the post-encoding sleep [3]. Sleep is thus hypothesized to mediate the reactivation of memories acquired during the encoding session [4,5] which are transferred from the hippocampus to the neocortex for consolidation [6-8].
Current research integrates behavioural, electrophysiological and pharmacological methods, to shed light on the modulatory effects of sleep and wakefulness on memory. Sawanglit et al. rectify our understanding of the involvement of the hippocampus in the consolidation of a type of memory previously thought to be hippocampus-independent during sleep [9-11]. The authors report that the long-term consolidation of both hippocampus-dependent-and-independent memories (H-dep & H-ind) requires neuronal replay during post-encoding sleep.
History
Insights into the role of the hippocampus in memory consolidation can be traced back to Patient Henry Molaison (H.M.), who initially suffered from severe epilepsy in the 1950s [12]. Locating the source of his epilepsy at the medial temporal lobe, both hippocampi were removed. While the surgery controlled his seizures, H.M. has intriguingly developed an anterograde amnesia (inability to commit new information to his memory), contributing to the development of the multiple parallel memory theory [11]. This theory proposes that central structures, notably the hippocampus, first receive various types of information, which are then transferred in parallel through specialised cognitive networks to be encoded in the neocortex. For instance, memory associating an object to a place (object-place recognition, OPR) is first stored in the hippocampus, which subsequently distributes it to the perirhinal cortex [13].
However, previous studies incorrectly concluded that the consolidation of novel objects memory (novel-object recognition, NOR) is independent of the hippocampus [14,15]. Behavioural procedures in these studies, including varying hippocampal lesion sizes and recovery durations, environment familiarity during encoding as well as the time of memory testing, might explain these discrepancies.
Behavioural results
Sawanglit et al. showed that the hippocampus enables the long-term consolidation of NOR via neuronal replay during slow wave sleep (SWS) [9]. The authors first confirmed previous research, indicating that immediate NOR relies primarily on the perirhinal cortex [13], and the suppression of hippocampal functions via a 2hr sleep deprivation or the injection of muscimol (a γ-aminobutyric acid receptor type A agonist) in the hippocampus, shows no effect on memory formation.
The encoding phase constituted a 10-minute interval during which the mouse interacts with 2 identical objects in an open field. To assess NOR, 1 of the 2 objects were replaced by a different novel object, and the discrimination ratio is calculated by:
Discrim. ratio = Time at novel-Time at familiar/ Time at novel+Time at familiar
Successful memory retrieval is shown by the mice exhibiting more interest in the novel object, as they would have remembered that they had interacted with the familiar object. Wakefulness was ensured by gentle handling with video recording, minimizing stress and possible confounding influences. Muscimol was introduced via surgically implanted cannulae (thin tubes) for the pharmacological inactivation of the dorsal hippocampus independently of behavioural states. Post-hoc results confirmed that muscimol was restricted to the desired area. The discrimination ratios obtained after 2hr and 1 week both showed no significant difference between control and hippocampus-inactivated mice, suggesting that the hippocampus is not involved in the short-term retrieval of H-ind memories. Additionally, hippocampal inactivation was suggested to facilitate H-ind memory performance as NOR tended to be slightly enhanced in muscimol-injected compared to control mice, confirming previous studies [15].
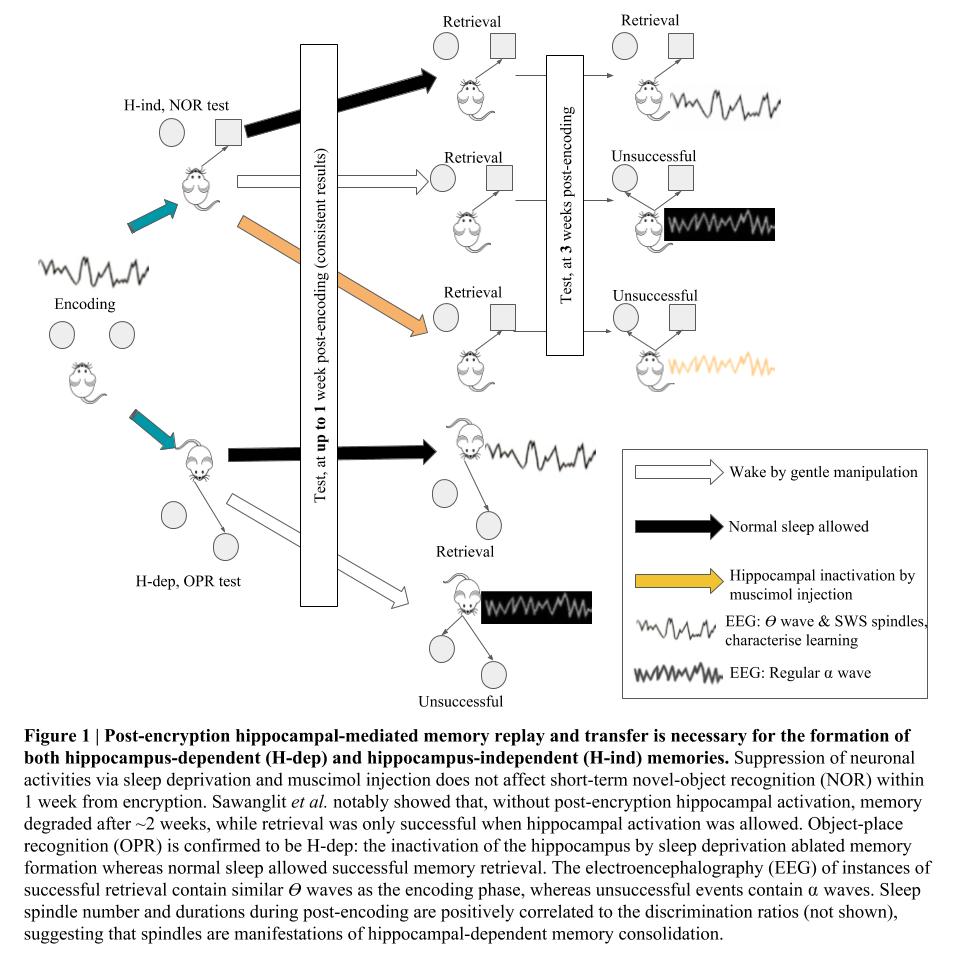
Importantly, the discrimination ratio obtained at 3 weeks post-encoding shows that inhibiting hippocampus causes significantly lower retention of H-ind memories, which is similar to H-dep memory consolidation patterns. This indicates that the effect of sleep on H-ind memory only emerges after 2 weeks post-encoding, corresponding to the duration for H-ind memories in wake conditions to fade. H-dep memories are formed when 1 of the accustomed objects is moved instead of being replaced, with the aim of testing OPR. Results indicated that sleep and hippocampal activities are necessary for OPR memory formation during any time post-encoding [13], which seem to indicate that OPR requires hippocampal cells activation like place cells.
Electrophysiological results
Beyond behavioural results, Sawanglit et al. identified electrophysiological correlates of H-ind memory formation and consolidation, advancing the neuronal replay theory (reoccurrence of neuronal activations that occurred during encoding). The discrimination of novel objects positively correlates with the number and duration of spindles, generated by the thalamocortical network during SWS, as well as theta oscillations during retrieval, generated by the septohippocampal circuit. Neural replay via hippocampal networks is quantitatively correlated to the transmission of memory information towards extrahippocampal networks. Muscimol injection post-encoding and sleep deprivation decrease spindle amount and duration. Using multiple surgically implanted electrodes to record the global EEG, local field potential and EMG (electromyography), the authors demonstrated that muscimol specifically affected the local field potential (local EEG) during SWS. These patterns suggest that sleep consolidates perirhinal-dependent object representations via hippocampus-mediated replay, which can be visualised through theta oscillations. Suppression of oscillations and replay mechanisms by inactivating the hippocampus ablates the long-term consolidation of memory of NOR, as well as the formation of OPR.
Relevance
This article provides a more accurate temporal framework for the hippocampus-neocortex transfer of memories during SWS. The consolidation theory from the 2-stage memory model [6] suggests that memory of episodes, like NOR, are initially encoded into hippocampal networks and, during consolidation, the representations are transferred to neocortical networks, which are long-term stores. This work adds that, for various types of memories (H-dep and H-ind), a short post-encoding sleep would allow hippocampal activation, neuronal replay and consolidation of long-term memory. While additional research is required to distinguish between the different subtypes of H-ind memories, which are not only limited to NOR [14], the current results can benefit clinical research [5]. Although not mentioned in Sawanglit et al.’s paper, it is tempting to stipulate that a short post-encoding sleep would enhance learning. If proven correct by future work in elucidating the molecular substrates underlying the neuronal replay theory, this discovery would have a substantial impact on sleep hygiene and culture in schools, where naps should be incorporated after selected lessons.
References
Editor-In-Chief:
Jayanthiny Kangatharan, PhD
Editors:
Sebastian Birtles, Jack Cooper, Mathew Herbert, Jayanthiny Kangatharan, PhD, Oriol Pavón Arocas, Fran van Heusden
By Anna Boake, PhD student in Neuroscience, University of Leeds
Autistic Spectrum Disorder (ASD) is a collective term for a range of developmental disorders affecting social interaction and communication which may negatively impact on school or work.
Neuroethics are moral considerations about the effects of neuroscience research on the human brain, with regard to the subject’s rights and wellbeing [1]. Eye tracking technologies used to detect autism in early infancy [2] discovered that toddlers with ASD spent substantially more time viewing geometric shapes rather than social images. Whilst useful for diagnosis, finding an appropriate way to explain to parents that their child has autism and what to do about it can be challenging and harmful [3]. A parent may react in an overly protective manner, which itself has been shown to increase anxiety in children [4]. Similarly, biomarkers such as a perturbation in blood serotonin levels can be used to identify ASD [5].
These advancements have greatly expanded our knowledge about the disorder and have contributed to the concept of neurodiversity, the belief that all forms of neurocognitive function are equally valid [6]. Although it proposes that ASD is a difference but not necessarily a disability, ethical challenges prevail.
For instance, well-being is arbitrarily defined as being in a state of health and happiness [7]. Stereotypic behaviours observed in autism such as flapping the hands, rocking back and forth and echolalia [8] are often viewed through a negative lens and judged as a sign of distress. However, these behaviours are often used by autistic individuals as a technique to self-soothe. This imposes the question that ensuring well-being of ASD people based on a subjective definition is potentially unnecessary and even unhelpful [9].
Fragile X syndrome is a genetic condition associated with ASD in 30% of patients. Drugs used to treat the former disorder are therefore being used separately to reduce the symptoms of ASD [10]. The effect of these drugs on a person’s identity must be considered and the how much interference is ethically appropriate. Savant syndrome occurs in 10% of ASD people, producing an exquisite skill in a particular area such as mathematics of drawing ability. If these skills are dulled from medication, are you taking away positive aspects of the disease as well as the debilitating ones? [10]
The success of manipulating autistic brains to ‘cure’ them of their abnormalities rests on society’s definition of normal; perhaps if neurotypicals change their beliefs and expectations, rather than changing people with ASD, they will integrate into society more successfully [11].
References
By Charlott Repschlager, PhD student in Neuroscience, King's College London
The field of neuroethics is a relatively new one, but considering that the use of pharmacological cognitive enhancement (PCE) among patients, as well as healthy people is increasing, it is gaining momentum [1, 3]. PCEs are drugs that can be used to improve certain mental functions in patients, including attention, learning and memory and some of the best-known example might be Adderall, an amphetamine often used by students as a “smart drug”. While PCEs can alter the way in which brain cells interact with each other, some PCEs have been shown to improve cognition in healthy, non-sleep deprived adults, which is where we leave the traditional patient medicine and enter the field of neuroethics [2].
In recent years the use of PCEs by healthy adults, especially students and academics, has markedly increased. Obtaining PCEs via someone else's prescription, the internet, or drug dealers leaves users vulnerable to side effects, addiction, and drug-drug interactions [1, 3]. Unfortunately, it is not fully understood how many PCEs work, given that cognition is complex and the ‘healthy’ adult range of cognition is very broad. PCEs, while improving cognition for some, may do nothing for others or even impair cognition. It is not clear what the long-term effects of PCEs are, and even less so what the possible effects might be in the still-developing adolescent brain [1].
If PCEs are risky, why take them? PCEs can be useful for those cognitively impaired by circumstances such as sleep deprivation and jet lag, and who still need to act at a 100%, such as surgeons or soldiers. Some people may even be self-medicating undiagnosed attention deficit disorders. This could potentially decrease social inequality in countries where health care is not accessible to everyone. On the other hand, social inequality might increase, as some people are able to afford PCEs, while others cannot [2, 3, 4]. Whether PCEs lead to social improvements or not, one may still ask what impact it will have on human values, including as hard work, creativity and reflection.
References
[1] Gaucher N, et al. (2013) Cognitive enhancement in children and adolescents: Is it in their best interests? Acta Paediatr 102 (12): 1118-1124.
[2] Mohamed AD & Sahakian BJ (2012) The ethics of elective psychopharmacology, IJNP 15 (4): 559-571.
[3] Mohamed AD (2014) Neuroethical issues in pharmacological cognitive enhancement. WIREs Cogn Sci 5 (5): 533-549.
[4] Shook JR, et al. (2014) Cognitive enhancement kept within contexts: neuroethics and informed public policy. Front Syst Neurosci 8:228.
By Anna Boake, PhD student in Neuroscience, University of Leeds
Child development encompasses the processes of physical, social and emotional growth that start at the time of birth and continue into late adolescence [1]. Whilst it can be beneficial to study this progression in order to understand development and identify abnormalities, this type of research raises with it a number of concern from the neuroethical perspective.
Firstly, medical imaging techniques such as MRI scans can present problems when unexpected abnormalities are observed, which occur in 10-13% of cases [2]. It can be difficult to determine whether the finding is serious enough to inform the parent, or if this will cause unnecessary grief i.e. a tumour may be found, but if it is benign it will not affect the child but may still invoke anxiety in some parents. Another imaging technique known as diffusion tensor imaging (DTI) can be used to assess the potential cognitive impairment of a child [3]. This interpretation of results from MRI and DTI could affect the way a child is treated, altering their self-perception and resulting in a self-fulfilling prophecy; this is a theory that expectations of an individual will influence and fulfil those expectations [4].
Another neuroethical concern is brought about by the use of biomarkers in the research of addition tendencies. The histone gene H3K9me3 acts as a tag to indicates a susceptibility to addiction, which could influence authorities’ decisions about allowing the use of potentially addictive substances by persons carrying such markers, which could segregate society. Alternatively, a person may excuse their behaviour regarding addiction as a fault of their biology [6].
In order to adhere to the ethical guidelines of research, informed consent, which asks for the approval from the participant to participate once the full details of the study have been disclosed, must be obtained. In child development studies, however, a parent is given the main responsibility of consent and only after this, the child may approve [3]. This generates neuroethical concerns as the child might not be able to give their assent or dissent independently and may feel coerced by their parent(s) to agree.
Another component of ethical guidelines is confidentiality, which is the principle that information about the participant disclosed during the study is classified. However, this may be breached in child development studies if the researcher suspects the child or somebody else may be in danger [2]. It can be difficult and subjective to ascertain when it is acceptable to breach this guideline, which may in turn damage a child’s trust and honesty with the researchers.
In summary, child development studies can provide crucial information on brain functioning that can impact a parent’s decision regarding the way a child is raised and possible interventions. However, the findings are not without substantial ethical concerns, which must be carefully considered by all those involved who are able to do so, before a child participates in such research.
References
By Caroline Casey, PhD student in Neuroscience, University College London
Over 10 years ago, the future of regenerative medicine was revolutionised with the development of a new technology. In 2006, Yamanaka and colleagues were successful in converting human skin fibroblasts to pluripotent stem cells (iPSCs) through a technique termed induced pluripotency, where transcription factors are transfected into adult cells and reprogram the potency of the cell [1]. Being pluripotent, these cells have the ability to differentiate into any cell type within the body, including complex cells such as neurons [2]. Soon after this technique was published, scientists and healthcare professionals began to realise the potential uses of these cells for disease treatments.
The ethical and moral considerations that surround the use of embryonic or foetal stem cells do not apply to iPSCs, as the original adult cells can be extracted from the body in minimally invasive procedures [3] and still differentiate into any given cell type. iPSCs can be generated from multiple sources which are easily accessible from the body, such as adipocytes, skin, or even blood cells [4]. Excitement about iPSCs rise with the realisation that lab-grown organs for transplants are now closer to being a reality.
Ten years on, considerable progress has been made with regards to iPSC-derived transplants. Several pre-clinical studies have been completed demonstrating that peripheral organs such as the liver and heart can be successfully rescued after differentiated stem cells are transplanted [5–7]. The central nervous system is a slightly different matter due to the inherent complexity of the brain. Despite this, pre-clinical studies evaluating the success of transplantation of either iPSC-derived neural stem cells or mature iPSC-derived neurons, have now been completed for multiple diseases including Alzheimer's disease and Parkinson's disease [8] Indeed, Japan’s Kyoto University is forging the way for Parkinson's disease; on November 9th 2018, surgeons injected iPSC-derived dopaminergic neural precursor cells into a human patient for the first time, and the regenerative medicine field is eagerly awaiting the follow-up results.
However, whilst the ethical considerations regarding the source of the cells seem sound, there are other moral questions to consider. How and for how long cells can be grown in the lab before it is deemed unethical is the first. Several groups have developed techniques whereby iPSC-derived neurons are grown in 3D, creating structures called cerebral organoids sometimes termed ‘mini-brains’ [9]. These mini-brains are capable of communicating within themselves; the neurons from different regions within the structure send action potentials and elicit responses, as well as having the capability to respond to external stimuli. For example, Boisvert et al. (2015) generated iPSC-derived nociceptive neurons that were capable of responding to painful stimuli. At what point do we define these mini-brains as ‘conscious’, and how do we as scientists measure that?
Another and slightly more complicated ethical question brings into discussion the ethics surrounding tissue transplantation, specifically neural tissue. We know that our brains, and their unique signature of activity, make us who we are. Our personalities, strengths, weaknesses and abilities are all determined at least to some extent by the functioning of our brain. So, what happens if you have a stroke that destroys the part of your brain that governs personality? If you were to have iPSC-derived stem cells transplanted to replace the damaged material, would your personality remain the same as before treatment? Or would the introduction of ‘new’ cells somehow change who you are? The opposing argument is that of course disease or trauma, and resulting loss of brain matter, would most likely change the behaviour of the patient anyway. Therefore, it could be argued that the risk is outweighed by the potential benefit of increasing the patient’s longevity.
It is also important to consider the ethics of stem-cell derived transplants, with regard to the patients’ ability to consent. As with all medical interventions, stem-cell derived neuronal transplants would have to undergo safety and efficacy assessments prior to widespread use [3,10]. However, unlike more traditional treatments that are tested on healthy volunteers first, in this case, the first patients to receive any transplant would be those afflicted with the specific disease being treated. Often, these patients would have some level of compromised cognition due to the nature of the disease being treated, such as neurodegenerative disorders. Consequently, their understanding of the procedure and risks entailed may be compromised. Furthermore, in order to assess the effectiveness of a treatment, a placebo or sham-treated control group is usually required. However, it could be deemed unethical for a patient to undergo a 'fake' surgery, or to have an inactive substance injected into their brain such as saline or dead iPSC-derived cells [3].
For the reasons here outlined, we must proceed with caution with regards to stem-cell derived therapies for neurological and neurodegenerative disorders. Whilst an incredible resource with huge therapeutic potential, iPSCs still raise ethical questions that must be addressed before their widespread use as treatments in the clinic.
References
[1] Takahashi K and Yamanaka S, (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell, 126 (4), pp. 663–76
[2] Pei D, Xu J, Zhuang Q, Tse H-F, Esteban M (2010) Induced pluripotent stem cell technology in regenerative medicine and biology. Adv. Biochem. Eng. Biotechnol., vol. 123, no. September 2012, pp. 127–141
[3] Barker RA and de Beaufort I (2013) Scientific and ethical issues related to stem cell research and interventions in neurodegenerative disorders of the brain. Prog. Neurobiol., vol. 110, pp. 63–73
[4] Wu S , FitzGerald KT, Giordano J (2018) On the Viability and Potential Value of Stem Cells for Repair and Treatment of Central Neurotrauma: Overview and Speculations. Front. Neurol., vol. 9, p. 602
[5] Rojas SV, et al. (2017) Transplantation of purified iPSC-derived cardiomyocytes in myocardial infarction. PLoS One, 12 ( 5), p. e0173222
[6] Nagamoto Y, et al. (2016) “Transplantation of a human iPSC-derived hepatocyte sheet increases survival in mice with acute liver failure. J. Hepatol., 64 (5), pp. 1068–1075
[7] Takebe T, et al. (2014) Generation of a vascularized and functional human liver from an iPSC-derived organ bud transplant,” Nat. Protoc., 9 (2), pp. 396–409
[8] Tang Y, Yu P, Cheng L (2017) Current progress in the derivation and therapeutic application of neural stem cells,” Cell Death Dis., 8 (10), p. e3108
[9] Lancaster MA, et al. (2013) Cerebral organoids model human brain development and microcephaly. Nature, 501 (7467), pp. 373–379
[10] Noel PR (1964) Clinical trials in the assessment of drug efficacy and safety. J. Coll. Gen. Pract.,7 (1), pp. 80–6
By Andrea Guerra, MD, PhD Fellow, Sapienza University of Rome
Tell us a little bit about yourself.
My name is Andrea Guerra. I am an Italian neurologist with clinical and research duties at Sapienza University of Rome. My field of interest concerns the management and treatment of neurodegenerative disorders of the central nervous system and, in particular, of Parkinson’s Disease and other movement disorders. Ever since I was just a student I have been attracted by the field of Clinical Neurophysiology. During my residency I focused on studying cortical neuroplasticity changes in physiological and pathological conditions by using various neurophysiological techniques, including transcranial magnetic stimulation (TMS) and TMS-EEG co-registration. In this context I also conducted some investigations as a Clinical Research Fellow at the Nuffield Department of Clinical Neurosciences - Experimental Neurology and Movement Disorders Group at Oxford, led by Prof. Peter Brown.
What is your line of research about and what are your highlights?
My current line of research actually started during my Fellowship in Oxford when we tried to better define whether and how transcranial alternating current stimulation (tACS), an innovative non-invasive brain stimulation technique, is able to modulate the brain rhythms, and influences the activity of the human primary motor cortex (M1). In that study we demonstrated that cortical β activity is related to cholinergic circuits involved in sensorimotor integration. Also, we provided evidence for a direct link between the phase of the ongoing β oscillations in M1 and the excitability state of the motor cortex. Starting from these encouraging results we then applied tACS, alone or in combination with TMS, to M1 with the aim of elucidating the role of brain oscillations in modulating cortical excitability, plasticity and motor behaviour. Overall, we found that tACS, delivered at the motor rhythms on M1 can influence the activity of specific interneuronal subpopulations, interacts with brain plasticity mechanisms and has frequency-dependent effects on motor behaviour.
What are the practical applications of your research?
The pathophysiology of several neurological disorders is presently far from being fully understood. Thus, a better understanding of the mechanisms underlying specific symptoms can lead to the development of more tailored pharmacological interventions in future. Also, some neurodegenerative disorders can be considered ‘oscillopathies’, where the alteration of physiological brain rhythms correlates with clinical deficits. This is the case, for example, in Parkinson’s Disease, where greatly enhanced β oscillations can be recorded in basal ganglia and correlated with bradykinesia and rigidity. Also, in Alzheimer’s Disease a widespread slowing of brain rhythms occurs and the power decrease of the α oscillations correlates with the severity of the disease. Finding a tool, such as tACS, that can reverse the pathological oscillations may represent a possible non-pharmacological therapy.
Who has inspired you during your research journey?
The patients. For a clinician and researcher like me I think the health of patients should be the first thought every day. Nowadays so many neurodegenerative disorders do not have any really effective treatment. Or, in some cases, treatments exist but they are complicated by the occurrence of side effects. Our job should be not only to treat but also to improve the current understanding of the mechanisms causing symptoms or disorders and to search for new and more effective therapies. Non-invasive brain stimulation techniques represent a useful tool in these regards.
Editor-In-Chief:
Jayanthiny Kangatharan, PhD
Editors:
Caroline Casey, Rachel Coneys, Daisy Hendley, Marta Huelin Gorriz, Pablo Izquierdo Garrudo, Oriol Pavón Arocas, Jayanthiny Kangatharan, PhD, Tiffany, Quinn, Ellena Sanderson, Fran van Heusden
By Edward Wickstead, PhD student in Neuroscience, Queen Mary University of London
For decades, amyloid beta (Aβ) and tau aggregates have been the primary neuropathological hallmarks associated with Alzheimer’s Disease (AD) and its clinical manifestations. However, some recent discrepancies have reignited the debate of whether Aβ or tau alone are enough to cause the extensive neuronal death seen at late stages of the disease. Today, considerable evidence supports the notion that neuroinflammation plays a role in the progression of AD (1,2), and two recent studies have looked into the role of the immune system in AD.
Macrophages to save the day?
For many years, excessive immune responses have been thought to exacerbate AD pathology (3), with immune cells infiltrating the brain leading to pathological inflammation and neuronal death. As a result, the search for treatments has often sought the suppression of the immune response. However, research lead by Dr Michal Schwartz at the Weizmann Institute of Science in Israel and presented at the FENS Forum of Neuroscience in Berlin (4) might change this trend. By using a specific antibody to activate the immune system they managed to drive peripheral macrophages into the brain to digest the damaged tissue. The results, yet to be published, further showed that boosting immune activity improves memory and cognition in these mice, alleviating the progressive symptoms of AD. Their next steps will focus on optimising the antibody’s properties and adapting the treatment regime to move on to the next stage: a clinical trial with human participants.
Aspirin: Amyloid’s newest enemy?
Aspirin is one of the most widely-used medications in the world. It stimulates the production of transcription factor EB (TFEB), a known master regulator of lysosomal biogenesis. This may have relevance for AD since lysosomes are the intracellular compartments where products taken up by cells (including Aβ) can be degraded. In fact, in a new study (5), researchers at Rush University Medical Center (Illinois, USA) orally administered a low-dose of aspirin to an AD mouse model for one month, before evaluating amyloid plaque deposition in the brain regions most affected by AD. They suggest that aspirin-induced TFEB upregulation occurs via the activation of peroxisome proliferator-activated receptor alpha (PPARα), and conclude that oral administration of low-dose aspirin successfully alleviates amyloid plaque pathology in both male and female mice, with this effect being PPARα-dependent. Their research highlights a new function of aspirin in stimulating lysosomal biogenesis through PPARα, suggest possible benefits of aspirin in reducing amyloid pathology in AD, and provides further hope that modulating the immune system could be utilised in the fight against AD.
References
By Anna Cranston, PhD student in Neuroscience, University of Aberdeen
The largest European gathering of neuroscientists took place in July 2018 at the 11th FENS (Federation of European Neuroscience Societies) conference, this year held in Berlin, Germany. Over 23,000 neuroscientists from across 32 European countries gathered for five days, with a shared goal of sharing advances in neuroscience research and education. An event of this scale does definitely not disappoint in terms of quality and quantity of research, with over 56 symposia organised across eight separate sessions. Symposia topics ranged from pre- and post-synaptic alterations in late-stage Parkinson’s disease, neural circuits for feeding behaviours and oral memory formation, to utilising CRISPR/ Cas9 gene editing as a treatment for neurological disorders. The conference also included a number of special interest talks, which covered issues such as reproducibility of scientific results and the use of animals in research, as well as many networking events.
The conference opened with the Fred Kavli Lecture, presented by Tom Insel of Palo Alto, USA, who gave a talk on behavioural analysis through digital phenotyping. Professor Insel proposed new ideas on behavioural healthcare with an emphasis on redefining how healthcare providers will utilise technology to diagnose and manage brain and mental health disorders. One of the highlights of the conference was the world-renowned Brain Prize Lecture. In 2017 the prestigious prize was awarded to Peter Dayan, Ray Dolan and Wolfram Schultz for their multidisciplinary analysis of learning and reward mechanisms in the brain, and the implications of their findings for our understanding of human behaviours and diseases, such as gambling, drug addiction, compulsive behaviour and schizophrenia.
Another highlight was the European Research Area Networks Neuron Excellent Paper in Neuroscience Award, presented this year to Cristina Garci?a Ca?ceres of the Helmholtz Zentrum Mu?nchen, Germany. Dr Ca?ceres presented her research on how astrocytes respond to the metabolism- regulating hormone insulin, in addition to leptin. This allows astrocytes to contribute to the control of sugar transport into the brain, suggesting that glucose transport is an active rather than a passive process. The FENS Forum comprised many other useful events for early-career researchers and PhD students, including poster sessions, PhD thesis prizes, evening networking events and socials, and an interesting talk on alternative careers for neuroscientists, thereby guaranteeing the event was a great success for scientists across all disciplines and career stages.

FENS 2018 conference. Photo Credit: FENS / KENES.
By Ryan Stanyard, MSc student in Neurosciences, King's College London
Emerging bioscience technologies are seeking to address the limited nerve regeneration evident in pathologies such as Spinal Cord Injury (SCI) utilising a class of biomaterials known as hydrogels. ‘Hydrogels’ or ‘scaffolds’ are comprised of between 95-99% water (hence ‘hydro’), with constituents including natural polymers such as collagen, hyaluronic acid or chitosan or synthetic polymers such as poly-ethylene glycol or poly-lactic acid (3, 4). Due to their elastic and versatile nature, hydrogels were initially used to build early colloidal gels made with inorganic salts (5).
Biomaterial approaches to enhancing CNS repair have ranged from the use of mesoporous silicon rods to deliver drugs to injured tissues through to scaffolding neural stem cell (NSC) growth (1, 2), composed of various polymers to act as guidance scaffolds for nerve regeneration.
In the decades to come, these constructs have been refined for use in nerve regeneration and repair research. Many hydrogel materials are hybrids, taking advantage of low-toxicity, biocompatible biological substrates and tuneable, mass-producible synthetic substrates.
Figure 1. NSC’s in Hydrogel Mesh
NSC’s can be encapsulated in the hydrogel mesh, alongside therapeutic molecules such as interferon in the example above, or neurotrophins or other molecules of interest. Photo credit: Li et al., (2018).
Hybrid gels are useful in incorporating synthetic chains for payload release; releasing therapeutic molecules as the hydrogel breaks down in vivo over time, enhancing nerve growth, and potentially restoring a degree of function (8). This release principles also works for the release of NSC’s, which can be incorporated as part of pre-set hydrogel grafts (Figure 1) which are surgically implanted onto damaged tissue, or utilised as part of injectable gel suspensions (3), gelating in response to physiological pH or temperature. As these gels degrade, the cells are released and begin to develop from early neurosphere into neural cell types including neurons (Figure 2).
The stiffness of a hydrogel determines the cell types (or ‘payload’) it can support, with stiffer gels being more conducive to lineages such as astrocytes, whilst softer scaffolds are more suited to culturing more fragile cell types, such as neurons (6, 7). It is worth noting that NSC scaffold cultures contain relative proportions of neurons, astrocytes and oligodendrocytes (and other cell types) and the stiffness simply determines the relative cell-type proportions.
Whilst human clinical trials are still relatively distant, early in vivo research is showing efficacy in spinal cord repair in rodent SCI models (9). These technologies are being combined with other emerging technologies, such as 3-D printing, to create commercially-viable, tailored implants with potential for in vivo repair (10, 11). If successful, these technologies may offer a cost-effective means of treating SCI and other CNS pathologies.
Figure 2. Hydrogels for NSC transplantation
NSC’s incorporated into hydrogels can differentiate and grow into healthy neuronal populations, as indicated by co-localised nuclear (Hoescht) and neurofilament staining (β-tubulin). Photo credit: Stanyard, Keele University (2017).
References
1. Kim, J., Li, W., Choi, Y., Lewin, S., Verbeke, C., Dranoff, G. and Mooney, D. (2014). Injectable, spontaneously assembling, inorganic scaffolds modulate immune cells in vivo and increase vaccine efficacy. Nature Biotechnology, 33(1), pp.64-72.
2. Li, H., Zheng, J., Wang, H., Becker, M. and Leipzig, N. (2018). Neural stem cell encapsulation and differentiation in strain promoted crosslinked polyethylene glycol-based hydrogels. Journal of Biomaterials Applications, 32(9), pp.1222-1230.
3. Pakulska, M., Ballios, B. and Shoichet, M. (2012). Injectable hydrogels for central nervous system therapy. Biomedical Materials, 7(2), p.024101.
4. Shoichet, M. (2010). Polymer Scaffolds for Biomaterials Applications. Macromolecules, 43(2), pp.581-591.
5. Chirani, N., Yahia, L., Gritsch, L., Motta, F., Chirani, S. and Faré, S. (2015). History and Applications of Hydrogels. Journal of Biomedical Sciences, 4(2), pp.1-23.
6. Engler, A., Sen, S., Sweeney, H. and Discher, D. (2006). Matrix Elasticity Directs Stem Cell Lineage Specification. Cell, 126(4), pp.677-689.
7. Nalam, P., Gosvami, N., Caporizzo, M., Composto, R. and Carpick, R. (2015). Nano-rheology of hydrogels using direct drive force modulation atomic force microscopy. Soft Matter, 11(41), pp.8165-8178.
8. Mauri, E., Sacchetti, A., Vicario, N., Peruzzotti-Jametti, L., Rossi, F. and Pluchino, S. (2018). Evaluation of RGD functionalization in hybrid hydrogels as 3D neural stem cell culture systems. Biomaterials Science, 6(3), pp.501-510.
9. Hong, L., Kim, Y., Park, H., Hwang, D., Cui, Y., Lee, E., Yahn, S., Lee, J., Song, S. and Kim, B. (2017). An injectable hydrogel enhances tissue repair after spinal cord injury by promoting extracellular matrix remodelling. Nature Communications, 8(533), pp.1-14.
10. Gou, M., Qu, X., Zhu, W., Xiang, M., Yang, J., Zhang, K., Wei, Y. and Chen, S. (2014).
Bio-inspired detoxification using 3D-printed hydrogel nanocomposites. Nature Communications, 5, pp.1-9.
11. Chen, M., Zhang, Y. and Zhang, L. (2017). Fabrication and characterization of a 3D bioprinted nanoparticle-hydrogel hybrid device for biomimetic detoxification. Nanoscale, 9(38), pp.14506-14511.
By Jayanthiny Kangatharan, PhD, Postdoctoral Research Assistant, Harvard University
Language. It is the tool that we use to solve problems and advance culture through communicating knowledge, teaching and learning from others. What happens when we first learn a language? Early in life we appear to be able to differentiate between virtually all phonetic units in the languages of the world (1).
However, around the age of nine months, the infant brain adjusts to the continual exposure of the native language (L1) (2). Furthermore, after puberty there is an obvious decrease in the ability of an individual to acquire native-like proficiency of a second language (L2). The continuous process of neural commitment to the L1-specific speech patterns experienced early in life could account for the corresponding decrease in the ability to acquire another language later on in life (3).
For example, cross-sectional studies showed that while 6-8 months old English infants were able to distinguish between two Hindi consonant sounds to the same level as native Hindi adults, 10-12 months old infants had difficulty with this task. This result was also replicated when assessing vowel sounds (4, 5).
Does this mean that learning L2 later in life will limit your ability to attain native-like proficiency, and become fluent? Luckily, the answer appears to be no! Published findings found that some individuals who started learning L2 later in life reached native fluency. However, the alternative was also true; learning L2 early in life did not guarantee fluency (6, 7, 8). For example, a study, which compared the grammatical judgment of native English speakers, with that of Vietnamese people who had learned English early in life, found no difference in performance between groups (9). Despite this, the Vietnamese early learners of English also appeared to retain their native accent, which could thus be argued as not reaching native-like proficiency in the English language. One can therefore say that early exposure to a second language will not guarantee native-like proficiency.
In another study, native English speakers were asked to rate the English accent of speech samples that had been produced by Dutch individuals who started learning English around 12 years of age (8). Approximately half of the Dutch cohort was mistaken for native English speakers, suggesting that it is possible for late L2 learners to attain pronunciation akin to native speakers. This finding was further supported by a study in which individuals from all over the world (Russia, Bulgaria, USA to name a few) were assessed on their Hebrew accent. The study found that the age at which individuals learnt L2 (in this case Hebrew) was not directly correlated to perceived native-like accent (10). Thus, an early start in learning L2 is not a prerequisite in acquiring unaccented speech.
There is also physiological evidence, which has been published to support this concept. A group of adult participants were trained on an artificial language, and exhibited a similar pattern of brain activity to that observed in native speakers when they process their first language (11). Furthermore, there appeared to be little difference in brain activity when a syntactic violation, or language error, was processed. Both groups exhibited a double peak of brain activity, termed early negativity and late positivity (N400 and P600), when a syntactic error was encountered. Essentially, the automatic detection and correction of such errors during language processing, is similar between native speakers and L2 individuals.
This indicates that both early and late learners of a language make use of the same brain mechanisms during the processing of language. Further supporting evidence is provided by other event-related brain-potential (ERP) studies. It has been shown that both the brain areas activated (15), and ERP patterns evoked in fluent L2 users, are largely observed in native speakers as well (12, 13). Difference in these parameters were revealed, however, when native and non-proficient speakers’ processing was compared (14, 16).
The evidence that we retain our ability to learn speech in different languages over the course of life is good news for all of us who recently thought of taking up a second language. No matter your age, or which language you are hoping to learn, you can become a fluent L2 speaker if the ideal learning environment is created. Thus, when you train yourself intensively in perceiving and producing the sounds of the second language, show the motivation and enthusiasm to sound native-like, along with massive L2 exposure, it will be possible for you to achieve your aim of becoming a fluent L2 speaker.
References
1. Kuhl, P. K., Conboy, B. T., Coffey-Corina, S., Padden, D., Rivera-Gaxiola, M., & Nelson, T. (2008). Phonetic learning as a pathway to language: New data and native language magnet theory expanded (NLM-e). Philosophical Transactions of the Royal Society B, 363, 979-1000.
2. Johnson, J.S., & Newport, E.L. (1989). Critical period effects in second language learning: the influence of maturational state on the acquisition of English as a second language, Cognitive Psychology, 21, 60-99.
3. Kuhl, P.K. (2004). Early language acquisition, Cracking the speech code. Nature Reviews Neuroscience, 5, 831-843.
4. Werker, J. F., & Tees, R. C. (. (1984a). Cross-language speech perception: Evidence for perceptual reorganization during the first year of life. Infant Behavior and Development, 7, 49-63.
5. Werker, J. F., & Lalonde, C. E. (1988). Developmental Psychology, 24, 672-683.
6. Birdsong, D. (1999). Introduction: Whys and why nots of the critical period hypothesis for second language acquisition. In D. Birdsong (Ed.), Second language acquisition and the critical period hypothesis (pp. 1-22). Mahwah, NJ: Erlbaum.
7. Birdsong, D. (2006). Age and second language acquisition and processing: A selective overview. Language Learning, 56, 9-48.
8. Bongaerts, T. (1999). Ultimate attainment in L2 pronunciation: The ease of very advanced late L2 learners. In D. Birdsong (Ed.), Second language acquisition and the critical period hypothesis (pp.133-149). Mahwah, NJ. Erlbaum.
9. McDonald, J.L. (2000). Grammaticality judgments in a second language: Influences of age of acquisition and native language. Applied Psycholinguistics, 21, 395-423.
10. Abu-Rabia, S., & Kehat, S. (2004). The critical period for second language pronunciation: Is there such a thing? Ten case studies of late starters who attained a native-like Hebrew accent. Educational Psychology, 24, 77-98.
11. Friederici, A.D., Steinhauer, K., & Pfeifer, E. (2002). Brain signatures of artificial language processing: Evidence challenging the critical period hypothesis. Proceedings of the National Academy of Sciences, 99, 529-534.
12. Hahne, A., & Friederici, A. D. (2001). Processing a second language: Late learners’ comprehension mechanisms as revealed by event-related brain potentials. Bilingualism: Language and Cognition, 4, 123-141.
13. Steinhauer, K., White, E.J., & Drury, J. (2009). Temporal dynamics of late second language acquisition: evidence from event-related brain potentials. Second Language Research, 25, 13-41.
14. Ojima, S., Nakata, H., & Kakigi, R. (2005). An ERP study of second language learning after childhood: Effects of proficiency. Journal of Cognitive Neuroscience, 17, 1212-1228.
15. Perani, D., Paulesu, E., Sebastian-Galles, N., Dupoux, E., Dehaene, S., Bettinardi, V., et al. (1998). The bilingual brain: Proficiency and age of acquisition of the second language. Brain, 121, 1841-1852.
16. Dehaene, S., Dupoux, E., Mehler, J., Cohen, L., Paulesu, E., Perani, D., et al. (1997). Anatomical variability in the cortical representation of first and second language. NeuroReport, 8, 3809-3815.
Editor-In-Chief:
Jayanthiny Kangatharan, PhD
Editors:
Christopher Casson, Rachel Coneys, Jack Cooper, Jayanthiny Kangatharan, PhD, Tiffany, Quinn, Kevin Ray, Ellena Sanderson, Daniel Shao
By Ghalia Khan, Marketing Trustee, Brain Bee
Directed by founder Dr. Norbert Myslinski, the International Brain Bee is a worldwide competition that motivates students to learn about the brain, captures their imaginations, and inspires them to pursue neuroscience careers to help treat and find cures for neurological and psychological disorders.

Sheep brain dissection. Photo Credit: British Brain Bee.
Founded in 1999, more than 60 nations and 175 chapters are engaged in coordinating Brain Bee programs around the world, and this number is rapidly increasing. About 50,000 students participate across six continents every year, and more than 600 neuroscientists have been involved with organizing and judging the events. An Alumni Club has been established to sustain the global community of young scientists into their university and career tracts.
The Brain Bee competition platform is organized on three levels: local, national, and international. Local scientific institutions are licensed by the International Brain Bee (IBB) to carry out city-wide or regional events, engaging students from 14-19 years of age. The first-place prize winners are granted the opportunity to compete at the national level. The National Champions are, in turn, invited to represent their country at the annual International Brain Bee competition, which is hosted by different neuroscience organizations during an international conference.
The Btitish Brain Bee Comeptition format.
References
1. Kandel ER (2006) In Search of Memory: The emergence of a new science of mind (W W Norton & Co.: New York).
2. The Brain Prize Lecture. Available at http://www.thebrainprize.org/ [Accessed 6th January 2017]
By Dr Joshua Au-Yeung, MBBS, FY2 Doctor in Stroke Medicine, NHS Northern Care Alliance
Professor Robin Sengupta is a prominent neurosurgeon who has been dubbed “neurosurgeon of the millennium” by his peers. His achievements are many; most recently he has been the recipient of an OBE, as well as being awarded the Medal of Honour by the World Federation of Neurological Surgeons (1).
Robin was born into poverty in Chittagong, India. His family could not afford to send him to school, so he would read each and every book that he could get his hands on. Eventually he was able to pay for school by tutoring younger students. Robin soon defied all odds to gain a place in medical school in Kolkata, India. After graduating, he moved to study surgery in Newcastle-upon-Tyne, UK. He stayed in Newcastle for 51 years, working as a leading neurosurgeon, carrying out cutting-edge research and treating countless patients.
During his neurosurgical training, Robin became interested in cerebral aneurysm operations. An aneurysm is characterised by weakness in the walls of a vein or an artery. Aneurysms can be congenital or acquired through life and exacerbated by lifestyle factors such as diet, exercise, smoking and alcoholism. When the vascular wall components are weakened, the weak section can expand and “balloon”. The danger is that an aneurysm is prone to bursting or leaking. It goes without saying that the mortality and functional impact of a ruptured cerebral aneurysm can be very serious.
In a time where there was no protocol or consensus on how to manage aneurysmal subarachnoid haemorrhages, a life-threatening and often fatal bleed in the surface of the brain, Robin strived to improve our knowledge and management of these patients.
Robin travelled extensively all over the world, visiting many different neurosurgeons to observe the vast array of techniques and management styles of patients with subarachnoid haemorrhages. Through his research, he identified which common factors conferred good outcomes in aneurysm surgery. Robin used what he had observed to refine and develop his own novel technique. He then published a paper detailing 32 anterior communicating artery operations that he had carried out; his ability to complete the operations with a mortality rate of zero was unheard of at the time (2). Robin’s pioneering technique and positive outcomes led to referrals from around the UK and internationally.
After dedicating much of his life to the NHS, Robin wanted to fulfil his own vision for delivering high quality, affordable healthcare to people in India. He decided to return to Kolkata, the city that made him the doctor he is today, and established the Institute of Neuroscience, Kolkata (IN-K) (3). Today, the IN-K is one of the best specialty hospitals in India for treatment, education and research in the field of neurology and neurosurger.
References
1. Newcastle University Press Office (2016) “World-leading neurosurgeon receives Honorary Doctor of Medicine”. Available at: https://www.ncl.ac.uk/press/articles/archive/2016/07/robinsenguptahonorarydegree/ (Accessed May 2018)
2. Sengupta RP, et al. (1975) Quality of survival following direct surgery for anterior communicating artery aneurysms. Journal of Neurosurgery, 43, 58-64.
3. I-NK Institute of Neuroscience Kolkata (2018) “Man with a Mission”. Available at http://www.neurokolkata.org/man-with-a-mission.shtml (Accessed May 2018)
By Francesco Monaca, Undergrad student in Biomedical Sciences, University of Southampton
The mystery in question is sleep. Fruit flies (Drosophila melanogaster) placed on a spherical treadmill are offering insights into the mechanisms regulating this marvellous yet poorly understood biological process. Fruit flies have already played a paramount role in elucidating the circadian timekeeping system. The circadian clock dictates when we should go to sleep, according to environmental cues. The same model organism is now being studied to shed light onto the sleep homeostat, a second ‘controller’ which might explain why we need to sleep in the first place.
In Drosophila, a population of dopaminergic neurons projecting to the ‘dorsal fan-shaped body’ (dFB) of the central complex (a region running across the midline of the insect brain) has been observed to induce sleep when stimulated (1). These neurons are electrically active and inactive in sleep-deprived and rested flies, respectively. It is therefore plausible to believe that dFB neurons effectively act as a switch between quiescent and active states, with Dop1R2 receptors mediating the arousing effects of dopamine.
To test this idea and characterise the mechanisms underlying the dopamine-modulated switch, the behaviour of head-fixed experimental flies on treadmills was studied while wake-promoting signals resulting in dopamine release were delivered via optogenetics (2). The behavioural mark indicating that flies transitioned from sleep to wakefulness was a period of locomotor activity after at least five minutes of inactivity.
Interestingly, this research highlighted that optogenetic stimulation of dFB neurons resulted in their transient hyperpolarisation and concomitant awakening of flies. Both effects were mediated by dopamine interacting with Dop1R2 receptors.
Surprisingly, while single dopamine pulses silenced dFB neurons temporarily, prolonged dopamine supply switched these neurones to the OFF (inactive) state, in which they remained even in the absence of transmitter. The speed of transition between ON and OFF states suggested that the translocation of ion channels to the plasma membrane could effectively be the mechanism underlying this switch, accounting for the increased potassium conductances and subsequent hyperpolarisation of dFB neurones observed when flies wake up.
Two main types of channels are expressed in dFB neurons in their ON, electrically active state, namely Shaker and Shab. Currents associated with these two channels are downregulated when cells are switched to their OFF state by dopamine, whereas voltage-independent leak currents are upregulated through a channel termed Sandman.
Therefore, in response to dopamine, Sandman is internalised within the plasma membrane and its hyperpolarising current, along with the attenuation of Shaker and Shab, is responsible for the transition of dFB neurons into OFF state, triggering awakening of flies. The next big step for sleep researchers would now be understanding the molecular players influencing this homeostatic switch.
References
1. Donlea, J. M., Pimentel, D. & Miesenböck, G. (2014) Neuronal machinery of sleep homeostasis in Drosophila. Neuron, 81, 860–87.
2. Pimentel, D., Donlea, J. M., Talbot, C.B., Song, S.M., Thurston, A.J.F. & Miesenböck, G. (2016) Operation of a homeostatic sleep switch. Nature, 536, 333-337.
Editor-In-Chief:
Jayanthiny Kangatharan, PhD
Editors:
Khatsha Ali, Molly Campbell, Jessica Chadwick, Rachel Coneys, Anne Cranston, Hayley Earle, Marta Huelin, Aisha Islam, Jayanthiny Kangatharan, PhD, Rohan Krajeski, PhD, Melissa Large, Oriol Pavón-Arocas, Tiffany Quinn, Fran van Heusden
By Rafael Cobb, MSc Student in Molecular Neuroscience, University of Bristol
The 2017 Brain Prize was awarded by The Lundbeck Foundation (1) to Wolfram Schultz, Peter Dayan and Ray Dolan for their study of he role of dopamine in reward and learning. Surprised at and grateful for winning a ticket to see them talk, I headed to the venue: the Royal Society at the heart of London. As I was approaching the venue from the Mall, the view was impressive.
Professor Schultz gave the opening talk, and set the tone for the evening, modestly joking that ‘There’s a really great lecture, after my lecture’. In an experiment analyzing behavior in monkeys, Schultz observed that when presented with two options, one slightly riskier and more rewarding than the other, monkeys preferred the higher stakes option. This tallies with evidence that the dopamine response is greater in high-risk gambles. Economic modelling suggests dopamine codes for reward subjectively; stronger dopamine release is based on a greater than expected reward. However, when receiving an expected reward, dopaminergic (dopamine producing) neurons do not activate above baseline-meaning no overall increase in dopamine when the reward is expected.
Next to speak, Professor Dayan went on to couch the dopamine reward response in terms of reward prediction. He suggested that dopamine release guides learning by acting as a feedback mechanism, which therefore influences decision-making. Professor Dayan’s talk centered on the mathematical model he created explaining how this mechanism works. From a neuropsychiatrist’s perspective, Professor Dolan extended the concept to humans and further elucidated dopamine’s role in learning, providing evidence that increasing dopamine production improves reward-dependent learning, whereas reducing dopamine production impairs it. Dolan also suggested that, given that dopamine production slowly declines once humans reach adulthood, learning may become impaired because of a reduction in the ability of dopaminergic neurons to predict reward. Elaborating on points made in the previous talks, Dolan explained the amount of money does not affect happiness in gambling. Instead, the perception of happiness in gambling is based on whether the gamblers are performing better than their dopamine-producing neurons predict.
From a molecular neuroscientist point of view, my main takeaways from the event were the interdisciplinary nature of the research, the opportunity to hear how molecular work can be complemented by mathematical models and translational experiments, and the realization that had they not combined their expertise, these researchers would have not have reached their fascinating conclusion.
References
1. The Brain Prize Lecture. Available at http;//www.thebrainprize.org/ [Accessed 11th January 2018]
By Ash Chetri, Research Software Engineer, University College London
During my year as a Masters student at the university of Edinburgh, I was fortunate to be surrounded by a diverse and stimulating academic community in the department of Neuroscience. It wasn’t until I met Dr. Jane Haley, that I was given the opportunity to volunteer for an outreach event in Roslin, Edinburgh. As well as meeting Dr. Haley at the event, I also met Dr. Szu-Han Wang (PI of Wang Lab at the Centre for Clinical Brain Sciences).
The conversation I had with Dr. Wang about my undergraduate research project on implicit memory, truly cemented my decision to work under her supervision towards my MSc thesis. So naturally when Dr. Wang advertised a placement for an MSc project, I applied without any haste. To my relief, Dr. Wang kindly accepted.
From my perspective, the best part of the project was the opportunity to participate in collaborative research in Taiwan. But as expected, magnetic resonance imaging (MRI) research requires a wide knowledge of varied subject areas. Furthermore, there tends to be researchers from a range of backgrounds. For example, animal research is particularly important as it bridges the understanding between cognition and behaviour (psychologists/cognitive neuroscientists) with the physics and physiology of MRI (radiologists, engineers). Hence, the collaboration between various fields is absolutely key in MRI research.
Before setting off for Taiwan, for months I focused on the technical gaps in my knowledge by iterative trouble-shooting and rote-learning through best practices. Although the sheer novelty of doing awake-rodent fMRI research became growingly apparent through the limited number of specific tools and research papers available (compared to human fMRI research). Thankfully, my collaborator Sun-Lin Han (a PhD student at LMRR, Chang-Gung University) guided me through the technicalities of independent component analysis and dynamic causal modelling.
Not only was I working in a beautiful country, but also I made many friends in the lab; connections I am grateful for. Taiwanese people are friendly; I never once felt daunted, alone, or even hungry (the canteen was filled with delicious Taiwanese food). I would recommend anyone to visit or consider working in Taiwan or in any international institution that they may be considering when presented with the opportunity. The learning experience was rich, something that perhaps cannot be rivalled with any of my peers at the University of Edinburgh. This would be something I’d do again without a moment’s thought.

The Radiology Department at the host institution Chang-Gung University that is privately supported by the Chang-Gung Group contains several beautiful murals designed by the students of the university.
By Cristiana Vagnoni, PhD Student in Neuroscience, University of Oxford
The CAJAL summer school “Interacting with Neural Circuits” was held on 2-22 July 2017, at the Champalimaud Centre for the Unknown (Lisbon, Portugal), a state-of-the-art research facility named in 2012 the best place worldwide, outside the USA, to do postdoctoral work (1). This course is part of the CAJAL Advanced Neuroscience Training Programme, a partnership between five leading neuroscience institutions (FENS, IBRO, the Gatsby Charitable Foundation, University of Bordeaux, and the Champalimaud Foundation) to establish a core neuroscience training facility in Europe (2). “Interacting with Neural Circuits” intended to combine lectures with hands-on training to highlight the latest techniques to investigate neural circuits, ranging from viral tracing to all-optical circuit interrogation. Students were provided with enough practical experience to understand the techniques’ advantages and disadvantages, to interpret experimental data correctly, and to have the capacity to apply their learning in their home laboratories.
The first two weeks of the course were structured with morning lectures given by international leading experts of the field, including Profs. Winfried Denk, Mark Schnitzer, Kenneth Harris, and Michael Häusser, among others. Topics ranged from neuronal subtype identification and connectomics, to in vivo circuit dissection and behavioural modeling. One lecture, by Prof. Rui Costa, focused on animal experimentation, with reflections about scientists’ responsibility in conducting animal research and in communicating its utility to the general public. Afternoons and evenings were dedicated to intensive hands-on training on the broadest collection of techniques: viral neuronal tracing, in vitro and in vivo patch-clamp recording, high density in vivo extracellular recordings, fibre-optic fluorescence microendoscopy, in vivo calcium imaging, and all-optical circuit interrogation (3).
During the last week, students were divided into groups and worked on a mini-project to gain independent experience with these techniques. My project focused on the predictive features of the visual cortex, comparing juvenile and adult mice, using in vivo calcium imaging and extracellular recordings with Neuropixels probes. Besides highlighting cutting-edge science, the course provided ample time to interact with course-mates, teaching assistants, speakers, and course organisers through many social events, including two poster sessions, a football match, a surfing trip, and daily shared meals.
In only three weeks, I explored new topics, developed a deeper understanding of the field, and learned new in vivo techniques and analysis approaches. Whether you are a first-year PhD student or an experienced post-doc, I can definitely recommend “Interacting with Neural Circuits” as an incredible opportunity for scientific growth and for establishing an international network of highly specialised researchers.
References
1. The Champalimaud Foundation History, available at http://www.fchampalimaud.org/en/the-foundation/history/
2. About the CAJAL Advanced Neuroscience Training Programme, available at http://www.fens.org/Training/CAJAL-programme/About-the-CAJAL-programme/
3. Interacting with Neural Circuits Website, available at https://sites.google.com/site/interactingneuralcircuits/
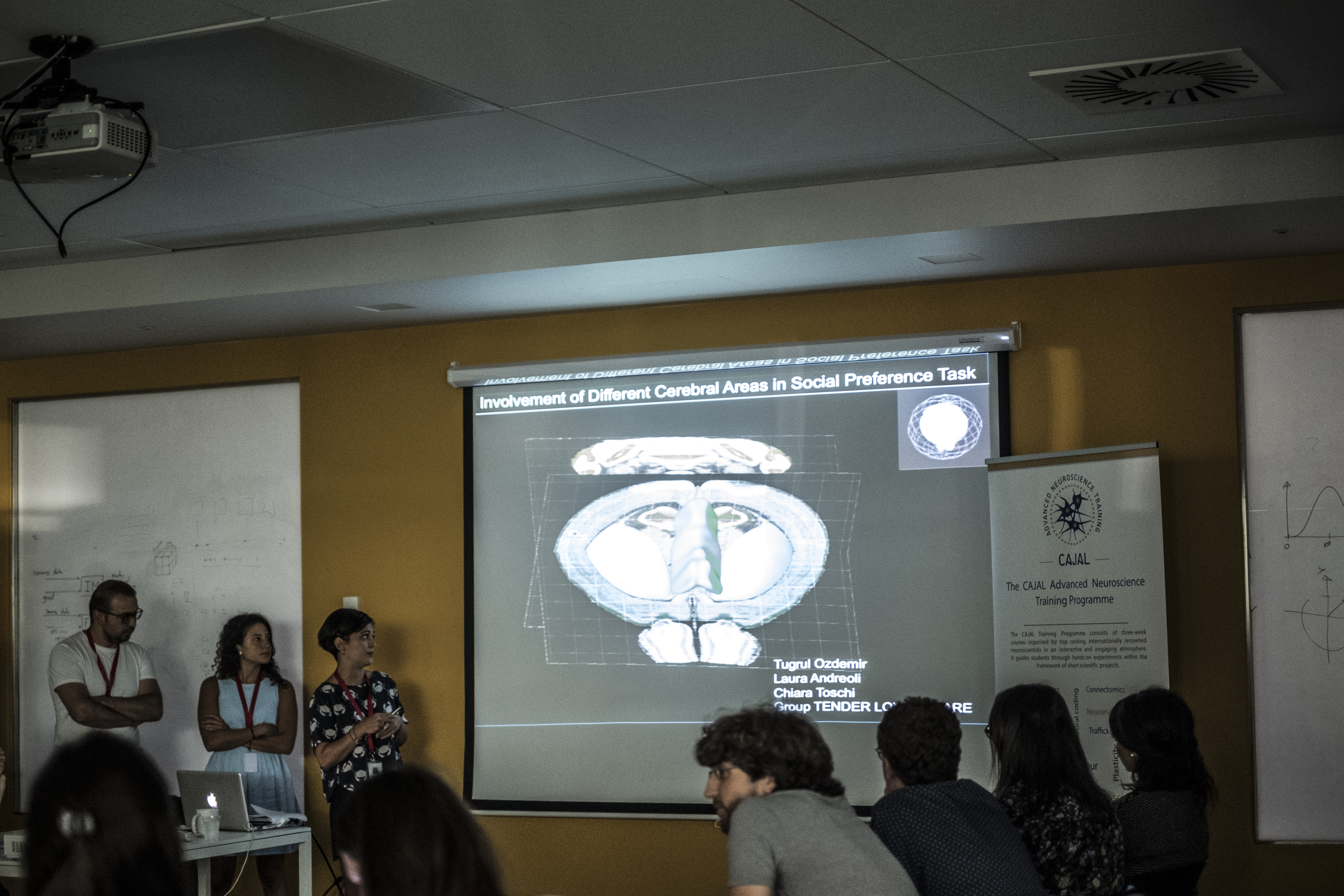
Final presentation of the mini-projects. Photo Credit: Catarina Ferreira da Silva.

Official group picture of the Summer School "Interacting with Neural Circuits", taken on July 21st 2017. Photo Credit: Catarina Ferreira da Silva.
Attendees’ opinions about the 2017 CAJAL summer school “Interacting with Neural Circuits”
“CAJAL-Interacting with Neural Circuits brought together outstanding researchers in key areas of contemporary neuroscience to discuss current concepts and define challenges for future research. Close interaction with the speakers, teaching assistants and course directors provided substantial benefit to my ongoing project and scientific development. At the end of the course, I returned to my lab very motivated and with full of ideas to implement.”
Tugrul Ozdemir
Division of Cognitive Neurobiology
Center for Brain Research, Medical University of Vienna, Austria
“It was amazing to be able to try a technique first person, to understand the advantages and disadvantages, the technical difficulties and success rate of the experimental approach. I can definitely say that I returned to my lab enriched in knowledge and with new ideas on which questions I can ask and what techniques I can use to answer these. We also had the possibility to meet other students and researchers in the field, which was fun and gave us the opportunity to network with a wide group of people from different fields in neuroscience.”
Chiara Toschi
Department of Psychology
University of Cambridge, UK
“I think the course was a great way to open our minds on the multiple resources and approaches available and left us with a fair amount of confidence we would be able to establish some of these techniques in our respective lab. Moreover, it was an incredible networking experience: I had the great opportunity to bond with students and researchers from all over the world, sharing experiences and ideas and setting the ground for future collaborations and good friendships.”
Luca Godenzini
Neural Networks Lab
Florey Institute of Neuroscience and Mental Health, Australia
“This Cajal summer school offered an amazing overview over the range of techniques today’s neuroscientists can choose from when tackling important questions. But it was much more than your typical lab course. Being part of the unique group of smart and motivated young scientists, we developed totally new ideas and made many new friends along the way. For me as a tool developer, it was a unique opportunity to experience first-hand, where new tools are needed or where current methods leave room for improvement. Looking back at how much we learned and how much fun we had together, I cannot believe it was only 3 weeks long.”
Manuel Alexander Mohr
Howard Hughes Medical Institute Janelia Research Campus, USA and
Department of Systems Science and Engineering, ETH Zürich, Switzerland
By Hayley Earle, MSc Student in Neuroscience, University of Glasgow
Iron is essential for many metabolic processes such as DNA synthesis (1). While variations in iron concentration exist throughout development, a general increase occurs with age. This is particularly marked in the substantia nigra (SN) brain region, which is involved in Parkinson’s disease (PD) (2,3).
This age dependent increase may be caused by an upregulation of the divalent metal transporter 1 (DMT1), a protein that transports ferrous ions (Fe2+) into cells (4). Research by Saadat and colleagues suggested a link between a polymorphism of the SLC11A2 gene (that encodes DMT1) and PD (5). Additionally, Song and colleagues determined that silencing of iron transporter, ferroportin 1, caused an elevation in intracellular iron levels (6), demonstrating its potential role.
Dopamine (DA) metabolism occurs via the monoamine oxidases (MAO-A and -B). In both aged and PD individuals, MAO-B is upregulated. Metabolism of DA through this enzyme leads to hydrogen peroxide (H2O2) production (7), which in turn can produce reactive oxygen species (ROS) through reversible Fenton and Haber-Weiss reactions (8). ROS are normally produced during aerobic respiration. Excessive production causes damage to proteins, lipids, DNA and RNA, and consequently induces cell death (9).
Ferroptosis is a novel iron-dependent form of regulated cell death that can be induced by a depletion of glutathione (10). This can occur through the inhibition of a glutathione-dependent enzyme, GPX4, which under normal physiological circumstances limits the rate of iron-dependent lipid peroxidation in cells (11, 12, 13). Ferroptosis can be prevented using ferrostatin and iron chelators (10). However, applying such conclusions to PD requires caution – most of the research into ferroptosis has been conducted in cancer cells.
There is currently very little research into PD associated ferroptosis. Do Van (14) determined that erastin, a ferroptosis inducer, can provoke cell death characteristic of ferroptosis, and confirmed Dixon’s findings that ferroptotic death is preventable by ferrostatin-1. In contrast to previous research, Do Van also reported GPX4 to be upregulated in PD, possibly due to co-localisation of GPX4 with neuromelanin, an iron chelator found in high concentrations within the SN (17). The SN is rich in dopaminergic neurons and iron, which may render it particularly vulnerable to the degeneration observed in PD (18, 19).
Whilst evidence remains limited, the implication of ferroptosis as the mode of cell death in PD presents a novel research direction, through which we may discover novel preventative or curative measures against this debilitating disease.
References
1. Abbaspour N, et al. (2014) Review on iron and its importance for human health. J Res Med Sci 19(2):164–174.
2. Bartzokis G, et al. (1997) MR evaluation of age-related increase of brain iron in young adult and older normal males. J Magn Reson Imaging 15(1):29–35.
3. Fukunaga M, et al. (2010) Layer-specific variation of iron content in cerebral cortex as a source of MRI contrast. Proc Natl Acad Sci 107(8):3834–3839.
4. Lu L-N, et al. (2016) Expression of iron transporters and pathological hallmarks of Parkinson’s and Alzheimer’s diseases in the brain of young, adult, and aged rats. Mol Neurobiol 54(7):5213–5224.
5. Saadat S.M, et al. (2015) Is the 1254T>C polymorphism in the DMT1 gene associated with Parkinson’s disease?. Neurosci Lett 594(1):51–54.
6. Song N, et al. (2010) Ferroportin 1 but not hephaestin contributes to iron accumulation in a cell model of Parkinson’s disease. Free Radic Biol Med 48(2):332–341.
7. Edmondson D (2014) Hydrogen Peroxide Produced by Mitochondrial Monoamine Oxidase Catalysis: Biological Implications. Curr Pharm Des 20(2):155–160.
8. Tabner, B.J, et al. (2001) Production of Reactive Osygen Species from Aggregating Proteins Implicated in Alzheimer’s Disease, Parkinson’s Disease and Other Neurodegenerative Diseases. Curr. Top. Med. Chem. 1(6):507–517.
9. Zorov D, et al. (2014) Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol Rev 94(3):909–950.
10. Dixon S.J, et al. (2012) Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149(5):1060–1072.
11. Gao M, et al. (2015) Metabolism and iron signaling in ferroptotic cell death. Oncotarget 6(34):35145–35146.
12. Cao J & Dixon S (2016) Mechanisms of ferroptosis. Cell Mol Life Sci 73(11-12):2195–2209.
13. Yang W & Stockwell B (2016) Ferroptosis: death by lipid peroxidation. Trends Cell Biol 26(3):165–176.
14. Do Van B, et al. (2016) Ferroptosis, a newly characterized form of cell death in Parkinson’s disease that is regulated by PKC. Neurobiol Dis 94(1):169–178.
15. Dolma S, et al. (2003) Identification of genotype-selective antitumor agents using synthetic lethal chemical screening in engineered human tumor cells. Cancer Cell 2003 3:285–296.
16. Zhang X, et al. (2014) Cell-based assays for Parkinson's disease using differentiated human LUHMES cells. Acta Pharmacol Sin 35(7):945–956.
17. Bellinger F, et al. (2011) Glutathione peroxidase 4 is associated with neuromelanin in substantia nigra and dystrophic axons in putamen of Parkinson's brain. Mol Neurodegener 6(8).
18. Gotz M, et al. (2004) The relevance of iron in the pathogenesis of Parkinson's disease. Ann N.Y Acad Sci 1012(1):193–208.
19. Ayton S & Lei P (2014) Nigral iron elevation is an invariable feature of Parkinson’s disease and is a sufficient cause of neurodegeneration. BioMed Red Int 2014.
By Adela Beloucif, Undergraduate Student in Psychology & Neuroscience, University of Glasgow
Fear and learning seem counterintuitive. After all, rarely are study sessions improved by feelings of terror. Learning that something is dangerous, or no longer a threat, is essential to the purpose of fear. Most fear is learned through classical conditioning, whereby animals associate a cue with something inherently dangerous or unpleasant. They can later learn something is not dangerous through repeated experience of that cue without trauma. This is termed extinction learning, whereby the new memory suppresses the original fear memory.
A recent study (1) investigated the potential neurons responsible for suppression of fear memories post extinction learning. They used transgenic TetTag mice expressing inhibitory DREADD (designer receptors exclusively activated by designer drugs) receptor hM4Di, to allow control of basolateral amygdala (BLA) parvalbumin-expressing (PV) interneurons, through inhibition of cell activity by injection of DREADD ligand clozapine-N-oxide (CNO). Mice were taught to fear a cage by receiving an electric shock, prior to extinguishing that fear through removal of the shock. Mice were then injected with CNO. Their fear response was measured as time spent frozen and immobile, using Actimetrics FreezeFrame software. Neuronal activity was measured through expression of ZIF protein and GFP. Both freezing behaviour and activity of fear-associated neurons in the BLA increased after CNO injection. Control mice were placed in a neutral box environment, receiving no shocks, and a vehicle injection of 5% DMSO in saline was used.
The authors predicted PV interneuron control to be due to greater levels of innervation between PV interneurons and BLA fear-associated neurons, compared with extinction behaviour associated neurons. However, the results of perisomatic analysis through use of the mCherry virus did not support this. To determine which other processes may potentially be influenced by PV interneurons, more hM4Di expressing mice were tested, and their Local Field Potentials (LFP) measured using surgically implanted electrodes. LFP oscillations between 3-6Hz were consistently linked with fear and freezing behaviour. As fear neurons were reactivated post CNO injection, the BLA LFP exhibited a shift from 6-12Hz towards 3-6Hz.
Ultimately, this research may be used for the treatment of anxiety disorders such as Post Traumatic Stress Disorder (PTSD), of which current treatments are largely ineffective in a number of patients (2). An understanding of exactly which neurons are involved in extinction learning may help develop more targeted drugs. Insight regarding brainwaves may also help, using neurofeedback training to alter brainwaves to help alleviate PTSD symptoms (2). Greater understanding of how these oscillations compete and interact could help improve treatment by allowing a more targeted approach.
References
1. Davis P, et al. (2017) Cellular and oscillatory substrates of fear extinction learning. Nat Neurosci 20 (11):1624-1633
2. Shalev A, et al. (2017) Post-Traumatic Stress Disorder. NEJM 376 (25):2459-2469.
By Stephen Eglen, PhD, Co-founder of the Special Interest Group 'Reproducible Research and Open Neuroscience' at the International Neuroinformatics Coordinating Facility
At first glance, it might seem odd that you would need to prefix the term "research" with the qualifier "reproducible". Surely, once you have a paper in your hands, you have all the details to reproduce someone else's work? That's certainly the theory when writing the paper, but often not the practice. Since 2004 we've set a problem for our masters students to reproduce key results from a paper within computational biology. Even though students carefully select a paper where the methods section seems comprehensive, and all the experimental data are available, they invariably find many missing details that preclude them from reproducing key figures or results. Many papers have been published on this failure to reproduce (1), commonly termed the "reproducibility crisis" (2). So, what might reproducible research entail? The definition varies across groups, but my interpretation is that when publishing result, labs should also provide all relevant datasets and methodology for transforming data into results. This means providing the spreadsheets or computational scripts to reproduce analysis. In turn, researchers should move away from "point and click" analysis methodologies (doing a t-test in Excel) towards computer scripts (such as R, matlab or python) so that others can re-run the same routines.
This leads us naturally to the second term, open science. The competitive nature of science, such as limited funding, jobs, and ‘high impact’ publications means that there is a natural tendency to withhold key datasets or analysis technologies: why give away your results to your competitors? An alternative view gaining prominence in recent years is that by sharing our resources, we allow others to build on our work and science as a whole should benefit. As an open scientist you are increasing your chances of making your work reproducible.
Being an open scientist may seem naive and altruistic, but there are selfish reasons for sharing your research (3). Many funding agencies now require data management plans for sharing of data post publication, and journals are increasingly asking for data and methods. My optimistic hope is that in 10 years we might be able to drop the qualifier ‘open’ and instead talk again simply about science.
Top tips for becoming an open scientist:
1. Read the guidelines in Markowetz (3) and think if they would apply to you.
2. Read about experiences such as Erin McKiernan.
3. Do experiments? Try writing a registered report before doing the experiments to reduce publication bias. https://www.nature.com/articles/s41562-016-0034
4. Talk to your local library to see what services they can offer to help archive and share your research. Find a local community of like-minded scientists!
5. Learn how to code, rather than using Excel, for your data analysis. e.g. www.datacarpentry.org
Comments? Send them to me twitter @StephenEglen
References
1. Ioannidis JPA (2005) Why most published research findings are false. PLoS Med 2:e124. https://doi.org/10.1371/journal.pmed.0020124
2. Baker M (2016) 1,500 scientists lift the lid onreproducibility. Nature 533:452–454. http://dx.doi.org/10.1038/533452a
3. Markowetz F (2015) Five selfish reasons to work reproducibly. Genome Biol 16:274. https://genomebiology.biomedcentral.com/articles/10.1186/s13059-015-0850-7
Editor-In-Chief:
Jayanthiny Kangatharan, PhD
Editors:
Inês Barreiros, Claire Chan, Jack Cooper, Harsha Gurnani, Jayanthiny Kangatharan, PhD, Josh Newman, Hope Oloye, Oriol Pavón Arocas
By Anna Stevenson, PhD student in Neuroscience, University of Edinburgh
The relevance of clinical research in establishing safe and effective medical therapies is well recognised. However, recently attention has turned to the scant evidence base from which to draw best practice in the treatment of medical conditions for a population that is typically excluded from such research: pregnant women.
Pregnant women suffer from the same medical conditions as the general population, but other than research on pregnancy itself, there is a dearth of clinical trials involving pregnant women, and few drugs are directly approved for use during pregnancy. Thus, doctors treating pregnant patients are forced to rely on inferences from drug studies that have explicitly excluded pregnant women in their trials. Women experience substantial physiological changes during pregnancy, including metabolic and haematological alterations, which are likely to impact on their pharmacodynamics. Extrapolation from such studies is, therefore, a vastly imperfect way of informing pharmaceutical dosage and efficacy, and doing so means pregnant women are in danger of under- or mis-treatment, and are ill-informed about the risk exposure to certain therapies could pose, both to themselves, and their unborn child. The need for a more informed understanding of therapies during pregnancy is compelling, and can only be achieved by including this population in clinical research. So why are pregnant women still so often excluded from clinical trials, and can the challenges facing their involvement in research be overcome? To answer such questions, we must first consider the key ethical principles of human clinical research.
The Nuremberg code, created as a result of the Nuremberg trials - a set of tribunals for war crimes in response to the horrific human experimentation carried out in Nazi concentration camps during World War II - was vital in establishing contemporary ethical principles for experimentation involving human participants. The code, along with the associated declaration of Helsinki, remains one of the main pieces of documentation upon which modern research ethics is based (1, 2). It sets out ten key requirements for medical research involving humans, including the necessity of voluntary informed consent - a cornerstone of modern medical ethics (see List 1).
|
List 1. Key requirements of the Nuremburg Code
|
Whilst the code sets out to protect the human rights of all research subjects, it is particularly relevant in the safeguarding of those who most need it: persons considered vulnerable. Historically, this group has included children, adults with compromised mental capacity, prisoners and pregnant women. Understandably, those who lack capacity, both physically and/or mentally, need this protection to ensure their rights remain inviolate and that they retain as much autonomy as the law allows. However, including pregnant women alongside this group is controversial as, unless they fall under another of the categories listed above, they are not a directly vulnerable group, retaining the same capacity for autonomous decision making, and are simply distinguished from the general population by being inseparable from a vulnerable ‘future person’ – their unborn child. It is the incapacity of this ‘future person’ maturing within their mother that has historically rendered the mother-foetus entity vulnerable in the eyes of clinical researchers and physicians. Generally, this has meant that even if a pregnant woman wishes to be a part of clinical research, she is likely to be excluded.
This classification is in contrast to official legalities surrounding the rights of pregnant women. Though widely discouraged, it is not illegal for pregnant women to smoke or drink alcohol, which can result in foetal growth restriction and developmental impairment, or indeed, in certain circumstances, to terminate the pregnancy. This means official UK laws permit the autonomy of expectant mothers more fully than the ethics surrounding their involvement in clinical experimentation. The reason for this difference is largely due to the fact that whilst the medical ethics surrounding foetuses is blurred with emotion and complexity (3-5), the actual UK law is more distinct: the rights of the foetus are not realised until the foetus is born alive. Legally, the foetus is a ‘person-in-waiting’, and this means that no litigation can be brought by a child against its mother for any pre-natal injuries or harm caused by their mother’s conduct before their birth [(6). While a mother may not be held liable by a judge or jury for harm caused to her child as a result of her gravid behaviour, clinical researchers have an enduring moral obligation to ‘do no harm’ to a future person in the course of their experiments, meaning pregnant women are routinely excluded from clinical trials to ensure their foetus is protected. This is an understandable concern, and the importance of preventing avoidable harm to women and their foetuses through research is undeniable. It is doubtful that anyone would argue that researchers should simply accept the same view as the law with regards to foetal rights in order to more readily include pregnant mothers in research; pregnant women cannot simply be treated like other research subjects as they are indeed physiologically different to the general population, and the safety of their baby is a priority. This may mean that involving them in research is more challenging and more considerations are needed; however, it seems there is a good argument for re-classifying pregnant women as ‘complex’, both scientifically and ethically, rather than ‘vulnerable’, when considering their participation. If this were universally agreed upon, it could lead to a corresponding shift in the inclusion criteria for clinical trials and an increase in studies that do not automatically exclude them due to their perceived vulnerability.
However, though this reclassification could aid trials to include pregnant women, there cannot be a universal answer regarding their involvement in clinical research as not all clinical experiments are alike. Medical research is broadly split into either therapeutic or non-therapeutic subgroups. Therapeutic research is conducted with therapeutic intent, often with the aim of comparing one intervention with another. Conversely, non-therapeutic research is conducted specifically to answer a scientific research question or to obtain knowledge, which may contribute to the future development of a treatment, but that is unlikely to produce a diagnostic, preventative, or therapeutic benefit to current subjects. The two types of research raise both overlapping and separate ethical issues with regard to the involvement of pregnant women.
Non-therapeutic research subjects receive no direct benefit from their involvement and instead must be willing to put themselves at risk, albeit reasonable risk, to benefit science. This research is hard to justify in pregnant women as although the pregnant mother may be willing to put herself at risk in order to further scientific knowledge, she cannot make the same decision for her unborn child. There is great debate about whether or not she should be allowed to put her foetus at risk when there is no possible therapeutic benefit for either of them. It does seem that non-therapeutic research on a pregnant woman might be justified if the woman gives full, informed consent and there is known to be no risk to the foetus, or if the risks involved are minimal and comparable to the normal risks a foetus is exposed to. If the risk is greater than this, non-therapeutic research would be extremely difficult to rationalise; as the pregnant volunteer is not being harmed by being excluded from such research, except arguably in terms of their autonomy, their omission seems fair.
Therapeutic research is really where the key debate about involving pregnant women in research lies. This type research can only be defined as ethical if there is an uncertainty about the best form of treatment, and clinicians involved in this research must believe that the experimental intervention being offered is likely to produce as good, if not better, outcomes as current best practice. Currently, there remains extensive uncertainty about the optimal treatment of numerous conditions concomitant with, but not exclusive to, pregnancy, precisely because of the paucity of treatment trials in pregnant women, so this initial ethical hurdle is easily overcome. Therapeutic research in pregnant women can be further split into that which may benefit only the woman, only the foetus, or both. The latter two of these may, in most cases, be acceptable as long as the pregnant mother has freely consented to accept any risk to herself the intervention may pose, and if the potential therapeutic benefits to either her foetus alone, or the two of them together, outweigh the risks involved. If research may benefit the mother, but carries a risk to her unborn child (such as treatment for some cancers), this is when the potential for a maternal-foetal ‘conflict of interest’ can arise. Answers about how best to proceed in such situations are clearer if the mother has a disease or condition that poses significant risk to her life. When the potential benefits are more subtle, the right answers are harder to come by.
A prominent example of such a situation, although it cannot be classified as research in itself, has become a key factor behind the caution felt by researchers about including pregnant women in treatment trials. Thalidomide, first developed as a sedative by German pharmaceutical company Chemie Grünenthal, was serendipitously found to be an effective anti-emetic and, as medication ingestion during pregnancy was not strictly controlled during this time, was prescribed to pregnant women suffering with morning sickness from 1953 onwards. As the drug had not been appropriately tested, these women and their physicians were unaware the drug could pose a risk to developing foetuses. Only after an Australian doctor - William McBride - published a letter in The Lancet in 1961 was the full, devastating effect of the drug revealed. Thalidomide was found to be directly responsible for teratogenic deformities in children and led to the estimated death of approximately 2,000 babies worldwide, with a further 10,000 suffering serious birth defects (7).
The shockwaves the scandal produced in the pharmaceutical industry are still being felt, with litigation and compensatory claims continuing over 50 years later. It is a major contributor to the reluctance to test drugs in pregnant women and, as a result, the evidence base for drug profiles in this population remains lacking, and they are left to be treated with medications that would be classed as archaic in other branches of medicine. While the thalidomide scandal was devastating, and pregnant women should never be exposed to such ill-informed treatment again, it is essential to recognise that excluding pregnant women from research can also be harmful. Had appropriate studies of thalidomide been conducted in pregnant women prior to its widespread use, it is likely the harmful effects of the drug would have been recognised at an early stage, resulting in far fewer incidences of adverse pregnancies. The thalidomide saga is an example of what can go wrong when proper research is not conducted, but, paradoxically, it has become one of the key deterrents against doing research in pregnant women.
Moving forward, caution would obviously be needed when it comes to involving pregnant women in such research but, with thoughtful study design, and after initial safety and efficacy profiles have been established elsewhere, the risks involved can be minimised and great benefit can be gained.
There is a critical need for an industry-wide debate about the inclusion of pregnant women in therapeutic research. If a clearer ethical framework can be established for research during pregnancy and we can begin to view this population as complex rather than vulnerable, more trials will be able to both include, and be designed for, pregnant women. It is true that these studies will need to be prepared for a more critical scrutiny of their ethics and risk/benefit outcomes, but this does not mean researchers and physicians should be reticent to design and run such trials. Pregnant women, like all patients, need safe, effective, evidence-based treatment and this can only be achieved by involving them in medical research. In doing so, we can move away from protecting pregnant women and their foetuses from research, to protecting them through research.
References
1. The Nuremberg Code (1947). (1966) BMJ 313 (7070):1448.
2. Trials of War Criminals before the Nuremberg Military Tribunals under Control Council Law No. 10: Nuremberg October 1946–April 1949. Vol. 2. Washington, U.S.: Government Printing Office.
3. Foulkes, M.A., et al. (2011) Clinical Research Enrolling Pregnant Women: A Workshop Summary. Journal of Women's Health 20 (10): 1429-1432.
4. Lupton, M.G. & Williams, DJ (2004) The ethics of research on pregnant women: is maternal consent sufficient? BJOG 111 (12): 1307-12.
5. Macklin, R. (2010) Enrolling pregnant women in biomedical research. The Lancet 375 (9715): 632-633.
6. Mason JK, & McCall Smith, A (1999) Law and Medical Ethics. London: Butterworths.
7. Franks, ME, Macpherson, GR & Figg, WD (2004) Thalidomide. The Lancet 363 (9423): 1802-1811.
By Eli Kinney-Lang, PhD Student in Engineering, University of Edinburgh
Who says summer camp isn’t for adults? As a group of students from the University of Edinburgh, we were lucky enough to attend the Current Issues in the Neonatal Connectome course at the University Medical Centre, Utrecht from June 12-16. It was a remarkable week filled with engaging conversations, a constant feeling of being inspired by scientific advances, and the joy of experiencing the local culture in Utrecht.
Each morning, the course featured four different talks from scientists and clinicians around the world. These talks provided a platform for speakers to discuss their research, their motivations, and their personal aims for improving the life of one of the most vulnerable populations on Earth: infants. After each talk, there was time set aside for questions and informal conversation, allowing for a less structured and more open discussion to emerge. The wealth of information provided touched on topics ranging from the enzymatic and cellular scope to broader explorations of behaviour in children on the autistic spectrum. With such a range of topics, it is hard to single out just one standout presentation or conversation. When asked about our favourite parts of the course, what we took away from it, and what the highlights were, here were some of the answers:
“I had an amazing time in Utrecht, interacting with world class researchers in their own environment was a fascinating experience, and really allowed me to address some of my own scientific ideas and concepts with fresh paradigms.” – Eamon Fitzgerald
“The main thing I took away from the course was a rekindled enthusiasm for neonatal brain research, and a feeling of privilege at being involved in such a collaborative and worthwhile field.” – Lorna Ginnell
“One of the most remarkable aspects of the course was the ability for it to cover the link between the technical and clinical world, really getting to see how things are related across the two.” – Manuel Blesa Cábez
“The knowledge, enthusiasm and kindness of the faculty were outstanding. They organized and hosted a networking dinner for us on top of putting together a range of plenary sessions and workshops which presented a complete view of the pipeline from scientific discovery to the translation of research into improved outcomes for the babies and families we care for. I am very excited to see the results from the forthcoming trial of stem cell therapy for treatment of neonatal stroke, and loved learning more on foetal MRI imaging from the keynote speaker, Dr. Moriah Thomason. Also, I really loved stroopwafels.” – Gemma Sullivan
In addition to the presentation line-up, there were several options for two-day workshops organised as 2-3 hour sessions in the afternoons. The four main workshops focused on imaging techniques, including electroencephalography (EEG), Ultrasound/Near-infrared Spectroscopy (NIRS), Diffusion Tensor Imaging (DTI), and Functional Magnetic Resonance Imaging (fMRI). These workshops offered a deeper slice into the technical methodology underlying both the work presented in the morning talks as well as general connectome research. Thankfully, the workshops were designed well to suit a wide range of expertise among the attendees – they included introductions to theory and background, how the techniques are being used in current research, and even a practical component.
Another highlight of the course was the collaboration between the University of Edinburgh and the UMC hosts to coordinate one-on-one lab visits for each student from Edinburgh. Prior to attending the course, all students were asked to supply names of UMC faculty with whom they would like to schedule a lab visit. In some cases, spontaneous lab visits were offered by hosts to Edinburgh students in addition to the student’s original scheduled meetings. The willingness of all the UMC faculty to participate in these small, intimate conversations reflects the dedication of this course to truly provide a means for networking and building collaborations.
Also, on the first day of the course all student participants were divided into groups and assigned the task of developing a 5-10 minute mock-up proposa on a topic of their choice, to be presented on the last day of the conference. These pitches were evaluated and then given feedback by a panel of experts from the invited speakers. Inspiration from the week’s talks shone through the pitches, with many groups incorporating themes featured in the course. The student proposals ranged from monitoring how stress affects development in preterm babies to ways to improve recovery outcomes for perinatal stroke and using organoids for modelling early-life connectomes.
Overall, the UMC course provided a unique opportunity to learn and develop new skills, and to connect over shared interests. These shared interests are an important foundation for promoting creative thinking beyond the simple labels commonly attached to ourselves and our work – A Scientist; A Psychologist; A Clinician; A Radiographer; An Engineer. That truly was the highlight of the course, and something which will not be readily forgotten.

Eli and fellow course attendees. Photo Credit: Aurore Menegaux
By Naomi Melamed, third-year biomedical sciences student at St. George's University of London?
Every year 300,000 people, mostly between the ages of 20 and 29, are diagnosed with a spinal cord injury (SCI). Such damage occurs when any number of the 13.5 billion neurons that make up the spinal cord die. Patients with SCI suffer a variable degree of functional loss to a particular muscle group, depending on the site and extent of injury. Unsurprisingly, these kinds of injury are associated with a range of sociological and economic effects, with 60% of patients being unemployed and 20–30% suffering from depression. This adds to the urgency to find a cure (1).
Over the past 20 years, many researchers have turned to mesenchymal stem cells (MSCs) as a possible treatment for SCI. MSCs are extracted from bone marrow and have the potential to differentiate into cells that make up bone, cartilage or fat. However, under the appropriate conditions, MSCs can also develop along neuronal lineages, making them a promising potential therapeutic treatment for replacing cells that are lost or damaged in SCI.
MSCs are favoured over stem cells of other lineages as they are anti-apoptotic, anti-inflammatory and anti-tumorigenic by nature. Trials are currently underway to investigate the viability of MSCs as an effective treatment. However, results to date have been mixed and researchers are also looking into alternative approaches.
For example, cell therapies for SCI might be more effective if they were based on transplantation of neuronal cells rather than undifferentiated stem cells. To this end, researchers have been developing methods to generate neuronal cells from pluripotent stem cells, such as embryonic stem cells or induced pluripotent stem cells (adult cells that have been converted into pluripotent stem cells). For example, a US–China collaboration have described a highly efficient method for generating motor neurons (2), while US researchers recently described a method for generating V2a interneurons and showed that they could integrate into the spinal cords of mice (3). Although many hurdles remain, these types of studies could one day provide a much-needed source of cells for treatment of SCI.
Therapeutic mesenchymal stem cell use in the central nervous system
Figure 1: Mesenchymal stem cells can differentiate into microglia and three types of cell that derive from neural progenitor cells. Each cell type produced contributes to the neuroregeneration process, depending on their lineage.
References
1. World Health Organisation (2013) Spinal cord injury. Available at www.who.int/mediacentre/factsheets/fs384/en/ [Accessed 7 May 2017].
2. Qu Q et al. (2016) High-efficiency motor neuron differentiation from human pluripotent stem cells and the function of Islet-1. Nat. Commun. 5:3449.
3. Butts JC et al. (2017) Differentiation of V2a interneurons from human pluripotent stem cells. Proc Natl Acad Sci USA 114:4969–74.
By Jack Cooper, Graduate student in Cells and Systems Biology, University of Oxford
Santiago Ramon y Cajal, viewed by many as the father of neuroscience, once said that “the brain is a world consisting of a number of unexplored continents and great stretches of unknown territory”. Whilst much is still unknown in neuroscience, it is safe to say that those continents have been better mapped, though there is still some way to go.
One of the major barriers to this progress is the opaque nature of the brain. Optical imaging methods cannot visualise tissue at great depths because of light scattering, resulting from differences in the rate at which light travels in water and fat molecules. Single-photon and two-photon microscopy can only image as deep as 50μm and 800μm below the brain surface respectively, preventing complete visualisation of global neural projection patterns and cell population positions. Instead, many thin brain slices have to be imaged and then reconstructed into 3D structures later – both time-consuming and costly. That is until the development of CLARITY, a Stanford-developed technology resulting in transparent brain tissue.
Following injection of both formaldehyde, used to fixed the tissue, and hydrogels, the CLARITY process then heats the brain and removes fats via chemical or electrical means. This leaves a transparent tissue-hydrogel mesh that retains the tissue’s original three-dimensional structure (1), whilst endogenous biomolecules such as neurotransmitters, proteins, and nucleic acids are fixed in place.
By visualising this three-dimensional structure of neurons, as well as the expression and localisation of mRNA, proteins, and neurotransmitters in the context of those structures, CLARITY allows us to extend our understanding of brains in normal and disease states. The treated tissue is both permeable to large molecules and hardy enough to be washed - this enables multiple rounds of antibody labelling of proteins and in situ hybridisation of nucleic acids. Unlike traditional approaches, this technique generates a high volume of information about local morphology, such as synapse type, and global morphology, which is incredibly powerful for understanding network dynamics.
In their seminal paper, the Deisseroth lab used the technique on both the mouse brain, and on sections from the frontal lobe of an autistic individual. By analysing long distance neural pathways, they discovered that neurons in this region had joined together to form abnormal ladder-like connections. Whilst such patterns had been previously suggested at by animal models of autism, CLARITY was able to demonstrate this in human tissue.
Since its inception, CLARITY has found great utility. It has been used to characterise many disease pathologies in three dimensions, including those involved with Alzheimer’s disease (2), multiple sclerosis and anxiety disorders, and is even being evaluated for applications in cancer and autoimmune disease diagnosis. In addition to this, the technique is uniquely positioned to better understand development, and the three dimensional movements and interactions of cells in the embryo. CLARITY is particularly useful for this as it can be used in a range of tissues beyond the brain, provided they aren’t too fibrous or pigmented; last year, for instance, the technique was successfully used to investigate the embryonic heart (3).
There are, of course, a number of disadvantages to CLARITY – namely the large start-up costs involved and the time taken for the clearing process to run, especially for larger tissue samples. This is all in addition to the time taken for immunostaining of such thick tissue, and an ~8% protein content loss that occurs during the process. However, despite this, it is still an exciting technological advancement towards understanding and relating brain circuit activity across multiple areas. So, even though CLARITY isn’t perfect, it holds great promise in helping us see through the mysteries of the brain.
CLARITY. Source: Deissoroth Lab.
References
1. Chung, K, et al. (2013) Structural and molecular interrogation of intact biological systems. Nature 497(7449): 332-337.
2. Ando K, et al. (2014) Inside Alzheimer brain with CLARITY: senile plaques, neurofibrillary tangles and axons in 3-D Acta Neuropathol 128 (3): 457–459.
3. Kolesová, H, et al. (2016) Comparison of different tissue clearing methods and 3D imaging techniques for visualization of GFP?expressing mouse embryos and embryonic hearts Hana Kolesová et al – using clarity on embryonic heart tissue. Histochem Cell Biol 146 (2):141–152
Editor-In-Chief:
Jayanthiny Kangatharan, PhD
Editors:
Joshua Au Yeung, MBBS, Inês Barreiros, Hannah Choi, Jack Cooper, Jayanthiny Kangatharan, PhD, Tamsin Nicholson, Patricia Rodriguez, Jamini Tharkar
By Emily Benn, undergrad student in Neuroscience, University of Keele
It is thought that humans developed language due to the vast, and complex, social capabilities we possess [1]. However, the question of how language evolved is difficult to answer as verbal sounds do not leave any physical fossils. Scientists believe that to tackle this problem we can study animals to which humans are closest on a genetic level -the great apes [2].
The great apes are a family of primates that include species of gorillas, chimpanzees, bonobos, orangutans, and humans. The intelligence of non-human great apes has been underestimated for a very long time and, ongoing research over the past 100 years has attempted to find out the extent of what these marvellous creatures are capable of [1].
The 1970s were when research in this area really started to develop. It was found that great apes have the capacity for language, but no means to use it. Due to physiological and neurological reasons, great apes cannot produce as many vast sounds as humans and therefore led scientists to question what would happen if great apes were taught to use sign language [3]. Great apes are the perfect candidates for the use of sign language as they have great control over the use of their hands, and impressive dexterity [3].
Some of the most famous research projects were Nim the chimpanzee and Koko the gorilla. During these projects, these great apes were taught to use American sign language. Nim was deemed to be an unsuccessful project as he failed to increase the length of his utterances after a 19 month period. However, Koko’s mean length utterances increased by over 33% in the same time period. 13% of Nim’s utterances were spontaneous compared to 41% of Koko’s. This disparity can be explained by the fact that Project Nim lacked organisation as he was taught by over 60 trainers (Koko was taught by 15 in total with 1 constant trainer) and as Nim grew older, he became a lot more aggressive [4].
Many scientists criticise this work as they believe that great ape language projects always fail. However, the line between success and failure is unclear and raises several questions. What do we mean by successful language acquisition? How many words in a sentence do they have to produce in order for it to be successful? Should the sentences be grammatically and structurally correct [3]? Many of these remain unanswered to this day. Nevertheless, research has moved on from trying to teach great apes language, to watching how great apes communicate with each other and, observing how complex this communication gets.
Despite the lack of consensus amongst the scientific community the fact that this kind of research has important implications is irrefutable. We can use the information gained from studying great apes to determine how humans might have developed language, and more importantly, what the main factors were that helped us to do so [1].
References
[1] Russon, A., Bard, K. and Parker, S. (1996). Reaching into thought. Cambridge: Cambridge University Press.
[2] Byrne, R. (1995). The thinking ape. Oxford: Oxford University Press.
[3] Wallman, J. (1992). Aping language. Cambridge: Cambridge University Press.
[4] Bindra, D., Patterson, F., Terrace, H., Petitto, L., Sanders, R. and Bever, T. (1981). Ape Language. Science, 211(4477):86–88.
By Leslie Smith, Professor of Computing, University of Stirling
Neuroinformatics combines neuroscience and informatics, aiming to develop and apply advanced tools and informatics-based approaches to interpreting neuroscience data, and enabling major advances in understanding brain structure and function.
Neuroinformatics has a long history in the UK. The UK Neuroinformatics Network was set up in 2004, becoming the UK Node [1] of the International Neuroinformatics Coordinating Forum [2] (INCF) in 2008. It was initially funded by the MRC. The UK Neuroinformatics London meeting in May 2016 [3] resulted in the first Neuroinformatics symposium at the BNA Festival that I organised on 12th April [4], and the BNA Neuroinformatics Special Interest Group (SIG) proposed by Professor Marcus Kaiser and myself [5].
The Neuroinformatics Symposium started with Marcus Kaiser introducing the SIG, showing how Neuroinformatics could aid clinical, experimental and engineering-based approaches to understanding, and even re-engineering the brain (https://tinyurl.com/BNAneuroinfSIG). I discussed why Neuroinformatics is critical. Sharing datasets (metadata and data), analysis tools and modelling techniques enables re-analysis of experiments, as well as comparisons across different tools and modelling techniques. Equally importantly they enhance reproducibility, a major issue for neuroscience.
Claudia Clopath (Lecturer, Bioengineering, UCL) discussed the onset and offset response in the auditory cortex. Interested in the causes and effects of LTP, and influences from environmental signals, she mixed experimental work with model-based data analysis, showing the importance of an informatics-based approach.
Tim Vogels (Henry Dale Fellow, Medical Sciences, University of Oxford) discussed what makes the “perfect synapse”. Interested in the effects of strengthening the pre- and post-synaptic mechanisms in the synapse, and in using models to examine the differences in these effects, he re-used data from 15 to 25 years ago, giving a real example of the importance of keeping data in a re-usable form.
Finally, Angus Silver (Professor of Neuroscience, UCL) discussed why modelling synapses matters. Synapse types in neural systems have very varied pre-synaptic spike rates. How does synaptic structure relate to function? He developed a repository for models of these and other neural circuitry, Open Source Brain [6] that enables model sharing, enhancing collaboration and re-use.
With over 100 delegates from different neuroscience areas attending, this reflects a strong and growing Neuroinformatics community within the BNA. Not only does Neuroinformatics provide a way of getting better value from experimental and modelling work, it helps with reproducibility, and this is critically important in future neuroscience.
[1] http://neuroinformatics.org.uk
[3] http://neuroinformatics.org.uk/Network2016/meeting03052016.html
[5] https://www.bna.org.uk/members/sigs/neuroinformatics/
[6] http://www.opensourcebrain.org
By Vinodh Ilangovan, PhD, Research Fellow, Max Planck Institute for Biophysical Chemistry, Göttingen, Germany
The EMBO EMBL Symposium on “Neural Circuits in the Past, Present and Future” in Heidelberg, Germany on 14th-17th May served as an excellent platform for over 100 neuroscientists across the globe to understand the structure and function of neural circuits from a new perspective. The purpose of this symposium was to enhance the exchange of novel ideas and methodologies among scientists who work on diverse model systems from the molecular to the psychological level to unravel the complexity of neural circuits. The scientific organizers of the symposium Detlev Arendt (EMBL, Germany), Richard Benton (University of Lausanne, Switzerland) and Leslie Vosshall (The Rockefeller University, USA) encouraged open scientific exchange, while the conference organizers Ana Karen Lopez Montero and Gwen Swanderson (EMBL, Heidelberg, Germany) supported the meticulous academic festival through an interactive framework.
The symposium was structured into eight sessions ranging from origin and evolution of nervous system; emerging animal and organism models to study neural circuits; innovation in neuroscience technologies; neural mechanisms of motivation and action to neurogenetics; and computational approaches to decipher neural circuits. Around 100 individual talks and posters investigating the structure or function of neural circuits were presented. Some of the highlights of the opening session included discussing the role of neuromodulation in persistent individual behaviours of invertebrates and the genetic basis of parental care evolution in mammals. The second day of the symposium focused on the beauty and elegance of neural circuits through discussions on the origins, commonalities and differences of animal nervous systems. Jean-François Brunet (École normale supérieure, France) emphasized the need to revise and revive the past understanding of autonomic nervous system, which remained elusive for centuries.
An exciting session on neurotechnologies opened up the possibility to evaluate techniques such as two-photon holographic optogenetics, dynamic mapping of neural circuits and functional ultrasound imaging in awake animals. Standard genetic model organisms such as the fruit fly, Drosophila and laboratory mouse dominated discussions on the understanding of circuits involved in decision-making, action planning and the emergence of complex behaviours. Special emphasis was put on the neural mechanisms of bodily self-consciousness and models of sensorimotor decision-making in humans. Computational approaches to understand how brains make complex decision with deterministic simple rules emerged as Leitmotiv of the circuit computations session.
Both organizers and participants ensured that time was devoted equally to inspiring talks and open discourse on research practices during coffee breaks and lunch. Poster sessions were very engaging and participants also had an opportunity to discuss rapid scientific communication methods such as preprints and open research platforms. Speed networking session held after dinner on the opening day of the symposium helped participants to step out of their comfort zones and foster interdisciplinary dialogues. A “Meet the Speakers” session during lunch break provided opportunity for early-career researchers to further discuss ideas and collaborations with eminent speakers. The evenings provided ample networking opportunities with an extravaganza of music and a picturesque backdrop of nature surrounding the conference centre. In conclusion, having facilitated knowledge exchange and constructive discussions, the symposium can be considered a great success. Organizers and participants alike have renewed enthusiasm and motivation, and are looking forward to next year’s symposium.
Supplementary information:
Participants of the symposium also tweeted to share their excitement about learning new insights into neural circuits with fellow scientists and tweeple across the globe. https://twitter.com/hashtag/eescircuits?f=tweets&vertical=default&src=hash
Photographs from the symposium:
Richard Benton welcomes delegates of the symposium. (Picture quality may be bad but no copyright issue)
These are pictures taken by EMBL photographer.

Welcome to neural circuits.

Speed networking.

‘Meet the speaker’ session.
Poster session.
Summary of the long talks
Cori Bargmann (The Rockefeller University, USA) opened the first session discussing the role of neuromodulation in persistent individual behaviors of invertebrates. Hopi Hoekstra (Harvard University, USA) discussed the genetic basis of parental care evolution in deer mice.
The second day of the symposium focused on the origins, commonalities and differences of animal nervous systems. Apart from interesting short talks, an exciting overview on evolution of neural circuits was presented by Leonid L. Moroz (University of Florida, USA), Detlev Arendt (EMBL, Germany), Gáspár Jékely (MPI for Developmental Biology, Germany), Paul Katz (Georgia State University, USA), Marcus Stensmyr (Lund University, Sweden) and Ralf J. Sommer (MPI for Developmental Biology, Germany). Jean-François Brunet (École normale supérieure, France) emphasized the need to revise and revive the past understanding of autonomic nervous system. Hillel Adesnik (UC Berkeley, USA) presented an exciting technique to perform two-photon holographic optogenetics. Alipasha Vaziri (The Rockefeller University, USA) shared insights into a novel approach to dynamically map neural circuits, while Botond Roska (Friedrich Miescher Institute for Biomedical Research, Switzerland) demonstrated the use of functional ultrasound imaging to study visual processing in awake mammals.
Understanding of circuits involved in decision-making and action planning is still dominated by standard genetic model organisms such as fruit fly Drosophila and laboratory mice. Gero Miesenboeck (University of Oxford, UK) discussed the how brains make decision with time as constraint. Kenta Asahina (The Salk Institute for Biological Study, USA) presented the neural basis of strategic action choice process involved in aggressive behaviors of fruit flies. Scott Sternson (Janelia Research Campus- HHMI, USA) focused on how neurons encode behavioral states like hunger and fear. The session on neural functions in humans spanned the neural mechanisms of bodily self-consciousness by Olaf Blanke (EPFL, Switzerland) and models of sensorimotor decision-making by Daniel Wolpert (Cambridge University, UK).
Marta Zlatic (Janelia Research Campus- HHMI, USA) showed how conflicting valances through memory generates behavioral choice in small animals like Drosophila larvae. Leslie Vosshall (The Rockefeller University, USA) presented novel insights into paternal enforcement of mating behaviors in mosquitoes. Silvia Arber (Biozentrum University of Basel, Switzerland) discussed how motor circuits generate precise behavioral movements. Claire Wyart (ICM, France) provided evidence for the involvement of cerebrospinal fluid neurons in mechanosensation and animal locomotion. Anthony Leonardo (Janelia Research Campus- HHMI, USA) presented in simplified manner how brains use set of rules to implement actions such as capturing a prey.
By Caroline Jahn, PhD student in Cognitive Neuroscience, Centre de Recherches Interdisciplinaires, Paris, France
From 9th March 2017 until 1st January 2018, the Brain Diaries exhibition is running at the Oxford University Museum of Natural History, charting research into the development of the brain throughout life. On the first floor of the glass roof, visitors can learn about how the brain learns and how it ages. Created in collaboration with neuroscientists from University of Oxford, this educational exhibition aims to captivate both adults and children by using a variety of media, from posters to fine-detailed 3D-printed brains. Even if you are unable to visit the museum in person, the online version will ensure the legacy of this thought-project (braindiaries.org).
However, the scope of the Brain Diaries project goes beyond this single exhibition. Numerous satellite events are being held across Oxford, encompassing talks, film screenings, science fairs, and even discussions within some of Oxford boisterous pubs. Regardless of your interest or background, there is certainly an event for you! The goal is to show people modern neuroscience in action by understanding current insights, and how research methods can be effectively applied to explore the unknown.
On 18th March my lab (www.waltonlab.org) participated in the “Brain Diaries Demos”. In our day-to-day research, we study brain chemicals such as dopamine. Dopamine often features in the media, but is frequently wrongly associated with pleasure and happiness (1). We therefore wanted to assess the degree to which this influences what people know about dopamine, and find entertaining ways of demonstrating what it really does. Under the attentive eyes of parents, children played our “Dopamine Dipper” marbles game and tried to discover which jar contained more red marbles to illustrate that dopamine provides a key learning signal in the brain. They also had a go at our “Heavy Feet Challenge” and decided if they wanted to put in extra effort to gain better rewards to show that dopamine is important for motivation and decision-making.
Overall, we all felt that the event provided a fun opportunity to remind ourselves that our research can and should be relevant and understandable to everyone. The very positive feedback from attendees gave us a great sense of accomplishment. The exhibition as a whole is already a triumph, with more than 15,000 visitors so far having enjoyed the chance to expand their scientific knowledge!
References
Editor-In-Chief:
Jayanthiny Kangatharan, PhD
Editors:
Natasha Gillies, Jayanthiny Kangatharan, PhD, Tamsin Nicholson
By Ana Bottura de Barros, PhD student in Neuroscience, University of Oxford
"... memory provides our lives with continuity. It gives us a coherent picture of the past that puts current experience in perspective. We are who we are because of what we learn and what we remember (1).”
This quote by Eric Kandel, from his autobiography 'In search of Memory', partially describes why I have pursued learning and memory as my field of study. It is for this reason that I have never felt so lucky in my life when the invitation from the Lundbeck Foundation to attend the Brain Prize Lecture (2) arrived via the BNA in my mailbox. The 2016 Brain Prize was given to 3 researchers that have shaped our understanding of how memory works in our brains: Tim Bliss, Graham Collingridge and Richard Morris - and I had the chance to see them talk.
The Lecture started with Tim Bliss who talked about how Long Term Potentiation (LTP) and transmission are linked. By explaining his experiments he was able to show that the chance of neurotransmitter release is increased with LTP induction. Then, Collingrigde illustrated the steps he undertook to explore the influence of Glutamate receptors on LTP, and see how different receptors were responsible for different phases of potentiation.
Just listening to these two talks was already a great experience. But as a behavioural neuroscientist, I would never have imagined that I would have the chance to hear Richard Morris describe how research linked LTP to behaviour. He talked us through O'Keefe's discovery of place cells, a finding that inspired his most famous work on the water maze. Next, he went on to show that Willshaw and Dayan's work on associative networks have important implications to linking LTP to memory representation. He could not finish without mentioning Whitlock's work on how learning induces LTP, thus linking LTP to behaviour.
Finally, to see a man who has achieved so much in his career tell us with great pride and excitement about his most recent work on dopamine release by the Ventral Tegmental Area (VTA) for novelty signalling should be inspiration enough for anyone in academia. And if that still was not enough, then the quick chat we had following the talks was a memory to be stored for a long term.
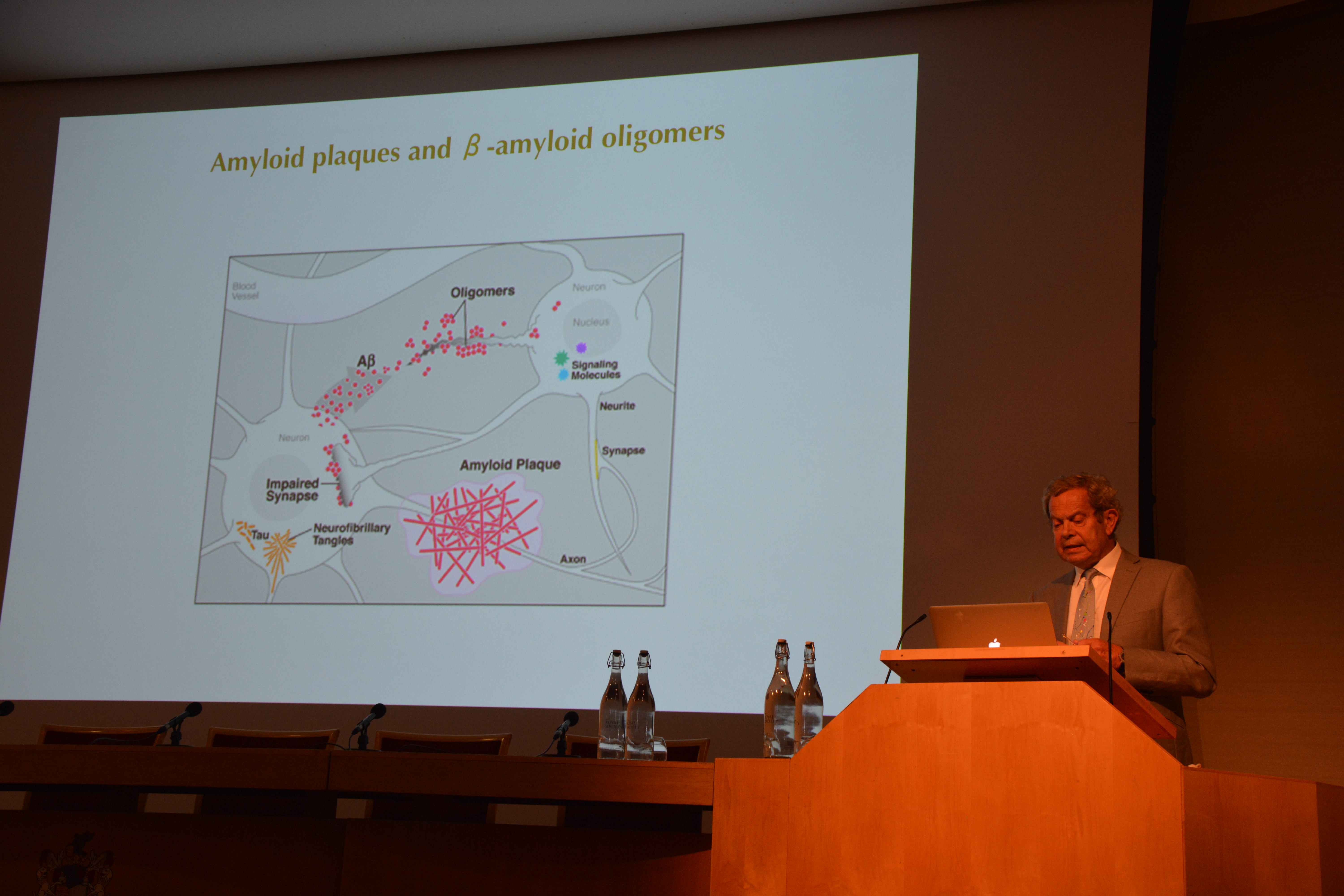
Richard Morris during his lecture. Photo Credit: Duncan Banks.

Richard Morris, Graham Collingridge, Tim Bliss Photo Credit: Duncan Banks.
References
1. Kandel ER (2006) In Search of Memory: The emergence of a new science of mind (W W Norton & Co.: New York).
2. The Brain Prize Lecture. Available at http://www.thebrainprize.org/ [Accessed 6th January 2017]
Pantoum By Jayanthiny Kangatharan, PhD, postdoctoral research assistant, Harvard University
In old age
Why did he steal memories?
He was not a criminal:
The symptoms of ageing
People saw one loss after another
He was not a criminal
The mind rested
People saw one loss after another
It was dark, and murky
The mind rested
Why did he steal memories?
It was dark, and murky
The symptoms of ageing
Haiku By Jayanthiny Kangatharan, PhD
In the mouse brain
Scanning ultrasound--
a promising procedure
to remove plaques
Double Tetractys By Jayanthiny Kangatharan, PhD
Little
I
Am small
And I do
Control posture
I receive inputs from the spinal cord
And integrate them to fine-tune motion
I hold eighty
Per cent of
All brain
Cells
Free verse By Jack Cooper, undergrad student in Cell and Systems Biology, University of Oxford
Outside View
What is a neuron? he asks,
his own soaked in the old-world glamour
of great masters, where Bach and Brahms
battle for remembrance, and for tribute.
Where notes sit on sheets, subjective,
teasing breath through flute to give
new forms, new revisions
of performance first heard lifetimes ago.
He asks, mind soaked with traditions
where interpretation is truth,
and truth is not tested.
What is a neuron? He asks,
and I understand he does not want answers
to the mundane questions of chemicals
spilling through clefts,
nor membranes that seep ions
like sap bleeding from bark.
He asks how his skin senses the quickening warmth
of the silver in his palm,
how his fingers and lungs dance on the razors edge
between music and disaster.
He asks how he can hear sound,
but feel beauty.
Rhyming Riddle By Jayanthiny Kangatharan, PhD
When life gets tough
Produced by a wide-ranging network
It will rise under a high load of work
Consistently correlated with complex mental tasks
It is also found to increase at key landmarks
Acts as carrier for cognitive processing across regions far apart
Try to guess this if you are smart
Diamante by Jayanthiny Kangatharan, PhD
Momentary awareness
Intention
Goal-driven, top-up
Endogenous cuing, covert orienting
Train of thought, flash of light in the Periphery
Exogenous cuing, overt orienting
Stimulus-driven, bottom-up
Instinct
Editor-In-Chief:
Jayanthiny Kangatharan, PhD
Editors:
Natasha Gillies, Tom Hall, Steven Jerjian, Jayanthiny Kangatharan, PhD, Andre Marques-Smith, PhD, Sophie Williams, Alicia Wilcox, Liam Wilson, PhD
By Jayanthiny Kangatharan, PhD
Between the 20th and 24th June the Pakeman Primary School in Islington launched its new Science Lab with a series of exciting events. On Monday and Tuesday children learned about volcanoes, rocks and minerals in the Lab. On Wednesday the children met Safari Pete who got them in contact with a variety of wild animals including a snake, scorpion and barn owl to teach the children about the importance of conserving wild life.
As an enthusiastic STEM ambassador I was thrilled at having been invited the following day to introduce two classes of 7-9 year old children to the wonders of the human brain! I met the teachers and set up my presentation. Five minutes later I introduced myself to 30 children aged 8-9 years and explained to them the purpose of my visit. I continued the session by asking the children what they already knew about the brain to gage their level of knowledge. To my surprise, I saw nearly all hands up. The children were clearly very eager to share their knowledge with the rest of the class! Specifically, they knew that the kind of food we eat can have a specific effect on our brain. Moreover, they were aware that the brain is responsible for acquiring information from our environment via our five senses. In addition, they knew that the brain enables us to move our bodies, and helps us to have a conscious mind.
Impressed by their level of knowledge, I proceeded to tell them about the concept of plasticity and clarified its importance in the process of forming new knowledge and modifying behaviour. I further emphasised how the children themselves can influence their own brain development by challenging their mental and physical abilities on a daily basis using simple but novel ways. Needless to say, the children already knew that studying is an essential activity to ensure a healthy brain development. I additionally pointed out that spending time in nature, acquiring a second language, and learning to play an instrument as well as adequate sleep, healthy nutrition and plenty of exercise can all positively stimulate their brain development.
After this interactive introduction into my presentation, I showed the children a few slides that illustrated the structure of the human brain from various perspectives. With the help of these slides, and couple of brain handouts, I then moved into the practical part of my visit. Here children built models of the brain using pink playdoh that was kindly provided by the teachers. I thoroughly enjoyed answering children’s questions about the brain during this practical part, and they happily chatted amongst themselves whilst coming up with creative ways of moulding the different structures of the brain. At the end of the one hour session I was pleased to hear the children’s overwhelmingly positive response to the interactive and practical activities of my session. I felt truly inspired to have been part of such a fantastic team that over the course of one week helped launch the new Science Lab at Pakeman Primary School.
I felt very honoured to have represented the subject of neuroscience within that team. All in all, I hope that I helped the children learn a little bit about their brains and how to take care of them. In future I am looking forward to seeing more primary schools opening a Science Lab that provides young children with the opportunity to learn about new ways of looking at the world in a positive, encouraging and supportive environment.
 Children wearing lab coats during the practical part of the session in their new Science Lab at Pakeman Primary School. Photo Credit: Jayanthiny Kangatharan.
Children wearing lab coats during the practical part of the session in their new Science Lab at Pakeman Primary School. Photo Credit: Jayanthiny Kangatharan.
By Joshua Au Yeung, final year medical student, Newcastle University
We stand in the midst of a revolution, the globalisation of urbanisation - one of the most pivotal social changes of the century (1). Cities and their populations are growing at an exponential rate and services are struggling to keep up, resulting in a host of problems, including overcrowding and struggles to meet basic needs of food, water, shelter, education and health. Eighteen out of the twenty-two most populated cities are now in developing countries and these cities will be impacted the most (1).
Kolkata, the medical, commercial and cultural hub of East India is a city that encapsulates the challenges of urbanisation and inequality. Boasting a population of 15 million (2), roughly four times the area of London and five times as dense, Kolkata is a city that is packed to its brim.
Merely 90 registered neurologists in Kolkata cater for the whole of East India and surrounding regions. It is not unusual for a neurologist to work seven days a week, averaging between 150-200 patients a day (3). This means that all their time is dedicated to clinical duty and none is left for quality scientific research.
Indeed, research papers published in India have little to no impact factor and 70% of papers are never cited. Research funding has stifled at a meagre 0.9% GDP for over 10 years (4, 5). However, doing research in India offers benefits that should not be overlooked: the large number of patients, rare untreated diseases, and the pathophysiological impact of poverty and malnutrition. As researchers abroad realise this, hence both funding and international collaborative projects are increasing (6). Expect to see a surge in joint research ventures from the western world, perhaps one that will match and benefit the growth of urban India.
A market in Kolkata. Photo Credit: Joshu Au-Yeung.
References
1. Gupta K, et al. (2006) Health and Living Conditions in Eight Indian Cities. Ministry of Health and Family Welfare document.
2. Office of the Registrar General & Census Commissioner, India (2011) Census of India: West Bengal.
3. Hrishikesh K (2016) Institute of Neuroscience- Kolkata. Interview conducted in January 21st, 2016.
4. Bala A & Gupta BM (2010) Mapping of Indian neuroscience research: a scientometric analysis of research output during 1999-2008. Neurol India. 58(1):35-41.
5. Shahabuddin SM (2013) Mapping neuroscience research in India – a bibliometric approach. Current Science. 104(12):1619-1626.
6. Nature Editorial. (2015) "A nation with ambition." Nature 521(7551):125.
by Joshua Au Yeung, MBBS, MRes, Foundation Doctor, Pennine Acute Trust
Clear, colourless and glistening; liquor cerebrospinalis, or cerebrospinal fluid (CSF), immerses our brain and spinal cord acting both as a cushion and a homeostatic buffer. Without CSF, not only would our neurones perish from electrochemical excitation, but our brains would collapse under their own weight, compromising blood flow, leading to a loss of cardiorespiratory function and coma.
CSF has a water-like consistency and composition like blood plasma: both containing a similar concentration of electrolytes and glucose. Litres of artificial, man-made CSF sit in various shaped beakers and flasks in the lab. Just like a chef, I concoct them using a carefully devised recipe.
The lab is a small, compact room filled to the brim with laboratory equipment, microscopes, computers and chemicals delivered from Britain. The lab sits on the top floor of a tertiary hospital: Institute of Neuroscience, Kolkata. Work is often disrupted by after-shocks from the tragic Nepal earthquakes reverberating through the building. The alarm rings, I quickly sprint down twenty flights of stairs to evacuate the hospital.
Neurosurgeons look for the eruption of sparkling CSF to mark the entry into the outermost layer of the brain: the dura. I wait eagerly by the surgeon’s side, with my beaker of cold artificial CSF. The patient is a 40-year-old gentleman suffering from epileptic seizures and headache from a large brain tumour, a “glioma”, measuring five centimetres in diameter. This is a rare sight; very few gliomas grow to that size without being detected and removed.
Once a piece of glioma is removed, it is carefully placed into the beaker, which is then locked into a secure box and oxygenated with a canister before being transported to the lab. The next step involves slicing the tumour into paper-thin slices and moving them into a pool of artificial CSF, recreating their physiological environment. Using sophisticated electrodes, neuronal activity and discharges can be measured in the tumour slices. I am interested in treating epileptic seizures deriving from tumoral glial cells.
Night falls and morning dawns. Experiments often take an arduous twenty four hours to complete as glioma tissue is rare. Not only is it prudent, but also ethical not to let any go to waste. With two slices yet to be tested, the peaceful silence is suddenly broken as a plastic cylinder drops onto the ground. The artificial CSF in the flasks oscillates and shakes violently; another aftershock. I stand up, finish my coffee and once again, prepare for my descent down the stairs.
by Joshua Au Yeung, MBBS, MRes, Foundation Doctor, Pennine Acute Trust
Several years have passed since research took me to the city of Kolkata, India. The experience highlighted the strength in research collaboration: how sharing knowledge, exchanging ideas and resources can help propel research forward. Since then, there have been many changes to the political and scientific climate in the United Kingdom. We are going to take a detour away from the east and examine the role that the UK plays on the global scientific stage. Political leanings aside, we ought to examine the likely impact of Brexit and what that means for researchers.
Statistically, the European Union is home to one fifth of researchers worldwide, yet generates over a third of all academic papers; an impressive 20% higher output than the US. Talented UK academics are free to collaborate with our neighbouring colleagues, with a reported 15-20% of staff in top academic UK universities originating from EU countries. These key collaborations have achieved some impressive feats including the Large Hadron Collider and the European Space Agency.
The UK receives an extraordinary amount of funding from the EU. The recent EU Horizon 2020 is an innovative research programme with ~80 billion euros of funding available over 7 years. There has already been 1.1 billion euros allocated to neuroscience research alone, with the UK being the top beneficiary. The aim of Horizon 2020 is to help fund research projects and reduce regulatory restrictions to help launch projects securely and quickly. Post Brexit, it is highly unlikely the UK will receive this level of funding.
The prevalence and cost of neurological conditions on the NHS are ever increasing given our ageing population. Dementia alone is estimated to cost the UK 26.3 billion pounds per annum. The EU joint programme Neurodegenerative Disease Research (JPND) is an organisation that attempts to improve our understanding and treatment of neurodegenerative conditions, such as Alzheimer’s and Parkinson’s disease, by funding large-scale studies, for example the cognitive function and ageing study (CFAS I & II). Other large research organisations include international collaborations such as the international initiative for traumatic brain injury research, allowing researchers all over the world to make breakthroughs and optimise the management of traumatic brain injuries.
The enormity of neurological disorders represents one of the greatest challenges we face in the 21st century. To tackle this problem, our collective focus should be to advance our cause by enriching our shared passion of research and maintaining our strong global collaborations. After all, we will achieve more together than we will by working alone.
What are your thoughts on the recent political climate and how has it affected your institution? We would love to hear your opinion! Please email any comments to j.auyeung@doctors.org.uk.
Editor-In-Chief:
Jayanthiny Kangatharan, PhD
Editors:
Inês Barreiros, Emily Benn, Tom Hall, Jayanthiny Kangatharan, PhD
By Alessandra Dillenburg and Owen Gwydion James
Young children in their final years of primary school become more active by playing team sports such as hockey and football, and learning how to ride a bike. Appropriate instruction alleviates the risk of injury when taking part in these sports, however, as exposure to injury increases, so too should an awareness of the type of damage individuals are susceptible to. While most adults understand that hurting your head is different to hurting your body, children may not yet be familiar with this concept. This is where getPROTECTED comes in.
Under the guidance of Dr. Jane Haley at Edinburgh Neuroscience, the two PhD students Alessandra Dillenburg and Owen Gwydion James recently set up a novel workshop on both brain function and head protection. This workshop is the newest addition to the getBRAINY (get Busy Running Activities Inspiring Neuroscience in the Young) series of workshops hosted by Edinburgh Neuroscience. The workshop aims to teach 10 to11-year-olds about the brain, how it works, and why it is so important to keep it safe. When explaining the functions of the brain in everyday life and the consequences of damaging specific brain areas, the two PhD students hope to convey to young children that the brain has a reduced healing capacity compared to the rest of the body, and highlight helmet safety as a preventative action to injury.
The inspiration for getPROTECTED came from a previous workshop headed by Alessandra at the University of Toronto and a mutual interest in cycling and public outreach: "We run a fun activity where the kids have to ‘pin the tail on the donkey’ by matching different senses to specific lobes. We talk about protective gear for different activities, as well as what bicycle helmets are made of and how they work. Our final activity has the kids run an experiment with the aim of testing how well different materials can absorb the energy of an object. Overall, we hope that getPROTECTED will be a fun, interactive workshop where children get to know their brains and understand why they need to protect them".
The workshop began this spring and aims to recruit volunteers from the Edinburgh Neuroscience community to train and send out to other schools in the following academic year. If the workshop is well received, the PhD students hope to develop a nationwide initiative for injury prevention in children.
If you’d like to discuss this or set up something similar at your university, please send an email at a.dillenburg@ed.ac.uk.
By Ksenia Kuznetsova and Vikoria-Eleni Gountouna
Between the 5th and 9th April 2016, the Nicodemus research group from the Institute for Genetics and Molecular Medicine at the University of Edinburgh organised a drop-in event entitled “Meet Your Social Brain” as part of the Edinburgh International Science Festival 2016.
The two-week festival, one of Europe's largest science festivals and the first science festival to be founded in 1989, hosted a large number of scientists from different disciplines ranging from biomedical sciences to chemistry and engineering to educate visitors about the recent developments in science, and to raise social awareness of the importance of science and its application to real life. The aim of the neuroscience drop-in event was to teach both parents and children about the social function of our brains, emotional intelligence and the relation of social skills to mental health.
 The drop-in event welcomed visitors of all ages. Members of the Nicodemus group and neuroscience student helpers chatted to children about brain anatomy and brain functions. While the youngest visitors got involved in creating anatomical brain hats, or emotional clocks that indicate one’s emotional state, older children, along with their parents and caregivers, enjoyed live neuroeconomic games and learned about the benefits of cultivating trust and cooperation in relationships. All games involved a reward of candy, proportional to the players’ winnings.
The drop-in event welcomed visitors of all ages. Members of the Nicodemus group and neuroscience student helpers chatted to children about brain anatomy and brain functions. While the youngest visitors got involved in creating anatomical brain hats, or emotional clocks that indicate one’s emotional state, older children, along with their parents and caregivers, enjoyed live neuroeconomic games and learned about the benefits of cultivating trust and cooperation in relationships. All games involved a reward of candy, proportional to the players’ winnings.
Adults and older children were also invited to an informal chat about ongoing research involving neuroimaging techniques, genomics and their role in mental health. Over the course of the festival data were collected as part of a pilot study to investigate developmental trajectories of socio-economic traits and the effect of family relationships on game play strategies. All in all, an estimated number of 2988 people visited the drop-in event during the 5 days, averaging almost 100 people per hour! Many thanks therefore go to everyone who helped make this event a success, with a special thanks to the mental health charity MQ, Edinburgh Neuroscience and its scientific coordinator Dr Jane Haley, and to all volunteers.
Image courtesy of Douglas Robertson Photography
By Julia Gottwald and Sally Jennings
On 17th March 2016, Cambridge Neuroscience organised the 28th Cambridge Neuroscience Seminar. Hosted by the Downing site campus, this meeting saw more than 300 delegates gather to attend a highly interactive symposium that showcased cutting-edge research on the theme of “New Directions”. The meeting was opened by Professor Bill Harris, head of the Department of Physiology, Development and Neuroscience. He thanked the seminar organisers, and especially Dr Dervila Glynn, for their hard work, and promised an exciting day of talks and posters.
The first presenter was Dr Rick Livesey who explained how the human cerebral cortex develops differently from other primates and mammals to contain more cortical neurons. This higher number of neurons is thought to be one of the reasons for our higher cognitive abilities. Next, Dr Lucy Cheke presented a new way of measuring memory in humans. She and her colleagues have developed an innovative spatial working memory task which was used to assess memory in obese subjects. They found that a higher BMI was correlated with impairments in memory, offering new insights into the relationship of eating behaviour and cognition. The final speaker of the first session was Dr Tiago Branco, who investigates instinctive behaviours. His research focuses on mice and their eating behaviour in threatening environments. Dr Branco explained how mice compute the decision to eat or escape in such environments.
Session two started with Dr Timothy O’Leary, who looked at an important challenge of our central nervous system: the need to maintain stable conditions and the ability to adapt to environmental changes. Dr O’Leary’s computational models show that organisms cannot be both perfectly stable and flexible: the option of flexibility comes at the price of less tightly controlled mechanisms. This talk was followed by Dr Kyle Treiber, who studied both neuroscience and criminology. She wants to develop a model of how and why individuals make the decisions to commit a crime, focusing on both biological and environmental factors. The session was concluded by Dr Sam Chamberlain who spoke about behavioural addictions. Traditionally, addictions were thought to be related to substances, such as alcohol or drugs. However, Dr Chamberlain argued that certain behaviours, such as compulsive gambling or stealing, show addictive characteristics. Individuals often crave the behaviour and show withdrawal symptoms when they abstain, similar to substance dependence.
The morning talks were followed by a lunch break and extended poster session. More than 60 researchers from Cambridge Neuroscience presented their recent research and had the opportunity to exchange ideas with other scientists. The posters covered the whole spectrum of neuroscience – from molecular and computational to cognitive and clinical neuroscience. Many delegates were also active on twitter during the lunch break and throughout the meeting. Tweets under the hashtag #CNS2016 allowed people from anywhere in the world to follow the symposium.
The afternoon session continued with a highly varied programme about predation, pain and psychosis. Dr Paloma Gonzalez-Bellido began the session by discussing hunting tactics of insects. She engaged the audience with flies’ optimal attack-trajectory, combing speed and acuity, using videos. Dr Ewan St John Smith continued to engage the audience with adorable videos of naked mole-rats. In contrast to other animals, naked mole-rats do not perceive acid as painful. Dr Smith is particularly interested in this acid-insensitivity of the naked mole-rat as a vehicle for understanding arthritic pain. Professor Paul Fletcher concluded the session with a presentation on psychosis. He argued that we can consider even our normal perception as a “controlled hallucination”: our brain tries to compute a model of the world with limited sensory information and ambiguity. This could serve as model for exploring psychosis, with false perceptions and irrational beliefs as inferential processing errors.
Professor Sarah Tabrizi, director of the Huntington’s Disease Centre at University College London, gave the Plenary Lecture. She discussed the great strides her team has been making in meeting the therapeutic challenge for Huntington’s disease. Huntington’s is a genetic disorder so Professor Tabrizi is focussing on a gene target. Indeed, her team has started work on the first ‘gene silencing’ trial in Huntington’s disease, a technique in which the expression of a gene is prevented. Afterwards, Professor Angela Roberts gave the closing remarks and the winners of the poster prizes were announced. The awards went to Matilde Vaghi for her poster on brain connectivity in OCD and to Dr Naotaka Horiguchi for his poster on the neural basis of contingency learning.
The conference was then opened to the public for a talk from Professor Giovanna Mallucci, University of Cambridge, about new directions in dementia treatment. Professor Mallucci’s new approach is to stave off neuronal loss, and associated worsening of dementia symptoms, by reducing the rate at which synapses are lost. Professor Mallucci’s approach considers that when mice cool and hibernate, their synapses are dismantled and reformed. Synapse recovery could be a valuable new direction for fighting dementia by trying to delay the symptoms.
The day ended with a drinks reception and exquisite conference dinner at Downing College. A worthy end to a popular meeting bringing together neuroscientists from Cambridge and beyond.
This report was first published on the CamBRAIN website.
 |
 |
 |
 |
Images courtesy of Cambridge Neuroscience.
By Dr Nicholas Irving
Public engagement can help you get a different perspective on your research and gain valuable communication skills. It can also raise the profile of your research area, which can sometimes contribute to maximising research impact. For these reasons, major funders nowadays will expect researchers to be active in public engagement; informing, consulting and collaborating with the wider public. Oxford has an active club for early career neuroscience researchers, the Cortex Club, founded in 2009. The Club’s main role is organising small informal discussions and debates on cutting-edge topics and significant, challenging issues in neuroscience. However, it also provides a fantastic forum where graduate students and early career researchers can work together on public engagement activities sharing their ideas and gaining experience.
A key part of the Oxford Neuroscience public engagement calendar is the Dana Foundation Brain Awareness Week held in March. This global campaign aims to increase public awareness of the progress and benefits of brain research. Brain Awareness Week 2016 was held from 14th- 20th March and the Cortex Club was as always keen to take part. Working with Silke Ackermann and Stephen Johnson from Oxford’s Museum of the History of Science, the students organised ‘Brain Aware’: a day of fun and engaging activities to inform the public about neuroscience research into a number of areas such as stroke and neurodegeneration. Graduate student Cristiana Vagnoni explained: “By using an established venue like a museum we knew the visitors would be ready and willing to be engaged. It also enabled us to take advantage of their communication channels to help promote the event. However, the programmes are drawn up months in advance and so you need to start planning early”. The students were also able to make use of the BNA Local Group Representative and Neuroscience Coordinator Dr Nicholas Irving who promoted ‘Brain Aware’ within the broader programme of Brain Awareness Week activities taking place across Oxford. This meant that they could benefit from shared publicity materials with a common design.
On the day running a public engagement activity can be very exhausting and so a small army of volunteers came along to help make ‘Brain Aware’ successful. As one volunteer, Emily Hinson, pointed out: “It’s very important to know who your target audience is so that you can tailor activities to suit them. We knew there would be a large proportion of families with children and so we made sure there was something for everyone”. Emily coordinated a range of demonstrations centred around research into stroke rehabilitation. These included throwing bean bags into a bucket while wearing prism glasses used to treat patients with hemispatial neglect. Students also brought along an EEG-based toy that is a fun way to introduce non-invasive imaging technologies and a brain stimulation kit which can be used to augment the beneficial effects of motor rehabilitation.
Students Lev Tankelevitch and Cristiana Vagnoni used ‘Brain Aware’ to try out some new activities including a cockroach leg experiment, which involved live recording of electrical activity from neurons inside a real, isolated cockroach leg. For this they used Spikerbox, which provides a simplified but complete recording setup fitting in the palm of the hand. Electrical signals from two pins placed inside the leg are amplified and displayed live on a connected monitor. This demo was set up as an experiment for the public to perform. Visitors used a magnifying glass to observe tiny hairs on the cockroach leg, which are connected to neurons inside and allow the insect to sense its environment. They were then encouraged to brush the hairs and observe the live neural response on the monitor – direct evidence of how sensory information is coded using electrical signals. They also used electrical current from the laptop to stimulate the leg and activate the nerve-muscle system, making the leg move and illustrating that neurons also use electricity to activate muscles and create movement.
In addition, these two students demonstrated a nerve-muscle interface experiment, in which they used a skin electrode to record the electrical activity in the forearm muscle of one volunteer, amplified this activity, and used it to electrically stimulate the nerve in the forearm of another volunteer. This (harmlessly) activated the muscle and made their fingers twitch – a strange and funny, but not painful, sensation. Essentially, one person's intentional muscle movement controlled the nerve (and muscle) of another, demonstrating that all nervous systems, including ours, use electrical activity to communicate.
Student Steven Chance used ‘Brain Aware’ to showcase an educational Xbox game about Alzheimer’s disease "Battle A.D." In this, visitors were able to battle tau mutants, collect stem cells and travel to the dentate gyrus to defeat Alzheimer's disease. The game was obviously particularly appealing to younger visitors (children and teenagers), familiarising them with terminology relating to brain anatomy and pathology. He also brought along a microscope with slides of human brain sections of Alzheimer’s pathology that had been cleared for public display. This enabled visitors to relate the cartoon game versions of pathology to the real-world examples through the microscope.
‘Brain Aware’ was aimed at everyone from age 6 and upwards and so student Sophie Avery brought along arts and crafts materials for children’s activities. These included making plasticine brains with different coloured cortical areas encouraging the young people to think about how different brain regions have different roles. Another activity which proved very popular was making pipe cleaner neurons. This helped visitors to learn about the structure of brain cells and how they link together into neural networks. Sophie commented “It is important to engage children in science from a young age, when they are curious and receptive. I really enjoyed explaining neuroscience to a range of children at the event, and was amazed by how perceptive and sharp they were.”
It is essential to perform an evaluation exercise to seek feedback from the public on what they thought of ‘Brain Aware’. This enables you to learn what went well and not so well and therefore constantly improve your public engagement activities. Therefore, visitors were encouraged to complete a short evaluation survey. The visitors’ interactions and feedback throughout the day were very encouraging and the results confirmed this. Visitor comments included: “Very engaging. A clear demonstration of how we produce and receive electrical signals. A lot of fun!” “Loved how you all took the time to explain to our little ones – thank you.” “Learned a lot about neurons and found it fascinating!” In the end the Museum counted a total of 672 people who visited ‘Brain Aware’. Summing up ‘Brain Aware’, Lev Tankelevitch and Cristiana Vagnoni said: "This was our first time independently organising a demonstration stall, and it couldn’t have been more successful. The enthusiasm, curiosity, and support that the visitors showed made the experience wonderful, and only strengthened our belief that scientists shouldn’t shy away from making their methods accessible to the public."
 |
 |
 |
 |
Images courtesy of Dr Nick Irving
By Inês Barreiros
‘To empower immigrant communities through science outreach’ - this is the motto of the ‘Native Scientist’, a non-profit organisation, which promotes science and language-integrated learning. Collaborating with international scientists, the Native Scientist organises science outreach sessions for bilingual school children with immigrant backgrounds.
In these sessions children have the opportunity to develop their multilingual skills by practicing a language that is not spoken in the country they currently live in. At the same time, pupils get to learn about science from real scientists and to know more about STEM-related careers. This gives voluntary scientists the opportunity to develop their communication skills while increasing the impact of their work through science outreach that is aimed at better social integration.
When it was first founded in 2013, ‘Native Scientist’ promoted sessions in London exclusively but it has now expanded. They are now organising UK wide science outreach sessions in the languages Portuguese, Spanish, French, Italian, Greek and German. They are reaching out to Europe as well by organising initiatives in Germany and France. Their aim is to increase both the number of school sessions and the range of languages covered.
I had the chance to participate in one of these wonderful sessions that was held at the City of London Academy in Islington. During this Portuguese class that was led by Dr Sara Marques, Dr Delfim Duarte, Dr Sara Trabulo and myself, the pupils learned about a range of scientific subjects including cancer, cell migration, disease-causing parasites and the workings of the brain. Despite their varying personal interests or hobbies, the children showed abundant amounts of curiosity and were interested in learning about the aforementioned scientific topics. They were also curious about what it is like to be a scientist and what motivates scientists to pursue their fascinating scientific endeavours. The opportunity to talk to children about my research and my passion for neuroscience turned out to be an incredibly rewarding and memorable experience, one that I can truly recommend.
With a number of sessions that are being planned for the next months, the Native Scientist is currently looking for more voluntary bilingual scientists. To get involved and have the opportunity to talk about your work and passion for science while inspiring young children, get in touch with the Native Scientist.
 |
 |
Images courtesy of NativeScientist 2016
Editor-In-Chief:
Jayanthiny Kangatharan, PhD
Editors:
Inês Barreiros, Natasha Gillies, Yuhua Guo, Katie Hoban, Davis Howett, Jayanthiny Kangatharan, PhD
By Jayanthiny Kangatharan, PhD
On Wednesday 17th February Queen Mary University of London hosted the inaugural research workshop on ‘Auditory Neuroscience, Cognition and Modelling’. This sold-out workshop brought together around 100 researchers from a range of neuroscience, cognitive and computational disciplines to discuss novel insights into the neuro-cognitive mechanisms of processing sound, speech and music.
Keynote lectures were delivered by Professor Elvira Brattica on the automatic and conscious processing of musical sound features in the brain; Dr Jean-Julien Aucouturier on the real-time transformations of emotional speech; and Dr Richard E. Turner on probabilistic models for natural audio signals. Additionally, attendees were captivated by six intriguing talks on such subjects as ‘EEG-based emotion detection in music listening’, ‘contextual influences on the neural encoding of speech sounds’ and ‘graphical modelling of neurological data in EEG/MEG’.
The one-day workshop included a poster session, in which 23 research posters were put on display, offering participants the opportunity to engage in a more in-depth exploration of the latest research in speech and music processing. Some notable highlights included ‘the adaptive effects of frequency on the auditory cortex’, and ‘the hierarchical nature of continuous speech processing’. By covering a wide diversity of topics, the session allowed attendees to gain a more profound understanding of how and why particular methods are used in current research. Topics ranged from ‘EEG-powered soundtrack for interactive theatre’ to ‘modelling transfer learning of polyphonic musical structure’.
Between sessions, participants enjoyed ample opportunities to network and socialize both during lunch and tea breaks and for a time following the workshop. Through this, successful collaborations could be cultivated across multiple disciplines. Numerous participants spoke favourably of the workshop and feedback was very positive, with one attendee commending the large number of consecutively high quality talks presented that combined ”strong theoretical grounds with methodological innovation”.
All in all, the innovative research-based event succeeded in bringing together researchers from a variety of disciplines including signal processing, auditory cognitive psychology and neuroscience. This opportunity for interdisciplinary collaboration will be an important step in establishing a coherent picture of what the brain is computing when it processes sound, speech and music. In view of the promising success of this first workshop, organizers are now considering establishing a workshop series that could be hosted by a different institution on each occasion. In addition, there is optimism for developing a research network that might be facilitated by such events. If you would like to know how you could get involved in the organization of the next research workshop on ‘Auditory Neuroscience, Cognition and Modelling’, send an email to wancm2016@qmul.ac.uk.
By Thomas Hallam
Choice of university is perhaps the most important decision I have had to make. Studying at the University of Southampton has greatly influenced my subject interests, determined my research experience, and has instilled ideas for a future career path I wish to pursue.
I am currently a third year Biomedical Science student hoping to study for a PhD in a neuroscience subject. I recently volunteered to help run a Sixth Form outreach event hosted by members of the University of Southampton Biological Sciences department and the Southampton Neuroscience Group. The aim of the outreach event was to show students what it is like to study Neuroscience/Biomedical Science at university, by educating them about the complexity of the human brain and research at Southampton to help us understand this fascinating organ.
The outreach day provided students with the opportunity to attend laboratory workshops, a university style lecture and talk with current undergraduate students, PhD students and academics about all aspects of university and neuroscience.
My role was to guide the students through the various workshops, which gave me a great opportunity to talk honestly and openly about studying my degree and studying at Southampton. It was fantastic to talk with students in a position that I had only been in three years before, and to be able to answer many of the questions that I had before coming to university.
I think that the purpose of outreach is different for everyone that attends. For some, the event was important in making students aware of Neuroscience as a potential degree/career option, and for others the day provided an opportunity to realise the diversity of the subject. But the importance of outreach, in my opinion, is to provide those attending with the knowledge to make an informed decision whether or not they want to study neuroscience by introducing them to both an unfamiliar and interesting environment. And based on the feedback received, it is clear that we were successful in our outreach event. One student wrote: ‘Biology/ Neuroscience didn’t originally interest me but today changed my opinion’.
I hope that Southampton and other universities can continue to provide outreach events to attract more students to pursue a career in neuroscience, and that as an aspiring neuroscientist, I am able to continue to promote the study of what I consider to be such a fascinating subject.
By Steven Jerjian
On 6th and 7th February 2016, Imperial’s South Kensington campus hosted LSNeuroN2016, the inaugural London Students’ Neuroscience Conference. The conference was organised by the London Students’ Neuroscience Network (LSNeuroN), a collaboration between the student-led neuroscience societies (NeuroSocs) at UCL, King’s, Imperial, Queen Mary’s, St. George’s Medical School, and now Goldsmiths. The sell-out event saw 400 students gather to attend a series of inspirational talks and workshops by world-leading academics and clinicians from across the field.
Keynote talks were delivered by neuroscientists John Donoghue, Sir Colin Blakemore, Maria Grazia Spillantini, and 2014 Nobel Laureate Professor John O’Keefe, on motor control, perception, neurodegenerative disease, and the hippocampal cognitive map, respectively.
Meanwhile, parallel-running symposium sessions organised by the member neuroscience societies of LSNeuroN, allowed a more in-depth exploration of a wide variety of topics. Over the two days, these included a neuropathology workshop featuring a live human brain dissection, panel discussions on neuroscience-inspired artificial intelligence and the interactions between art and neuroscience, as well as fascinating speaker-led symposia on neuro-oncology, neurodegenerative diseases, psychiatric disorders, traumatic brain injury, and more.
In addition, over 40 students presented posters on their own research, with prizes (including a free 1-year BNA membership) awarded for the best presentations in both PhD and non-PhD categories. Exhibitor stands gave students the opportunity to engage with companies selling neuroscience-relevant products during lunch and coffee breaks, and an evening wine reception on the first day encouraged plenty of mingling!
Feedback received has generally been extremely positive, with many students and speakers particularly impressed by the professional level of organisation of a student-led event, the breadth and depth of topics covered, and the quality of invited speakers.
Although the first conference organised on this scale for students, we hope that the great success of LSNeuroN2016 will lead to many more in the coming years, possibly on a biennial basis, bringing together more students and building on the success of this conference. If you are interested in finding out more about LSNeuroN or any of the neuroscience societies, then visit our website at lsneuron.com, follow us on social media, or send us an email at info@lsneuron.com!
LSNeuroN would like to thank all sponsors of this event, including the British Neuroscience Association, for their generous support and encouragement.
By Thomas Hallam
The four-year, integrated Master's in Neuroscience (MNeuroSci) course at the University of Southampton has been an engaging, fascinating and challenging experience. It has provided me with the opportunity to work with multiple PIs, research fellows and PhD students, all whilst being part of a faction of undergraduates striving to produce results, meet deadlines and somehow manage to enjoy the social side of university in parallel.
In our penultimate and final years of study we are trusted to manage our own time. I initially felt daunted by the weight of responsibility that a long lab project presents, but quickly got used to working under pressure and trying to steer the course of my own science by designing experiments and presenting my own data. The MNeuroSci course allows you to focus on the subjects that interest you most, which in my case is the investigation of neurodegenerative pathologies.
A major challenge of the MNeuroSci has been returning froma four-month summer to find an incredible number of milestones to be achieved in the final year if you are aiming to obtain a good grade. From my point of view, as aspiring neuroscientists, we have the most interesting selection of modules available to us. However, they are often also the most difficult, with a heavy workload that takes a while to adapt to, until after the first couple of months, when we really get into the swing of things.
A chief component of the MNeuroSci involves gaining a greater understanding of the complexity of the brain ans its function, both at the molecular and morphological level. It also focuses on widening our experience of scientific research in general. We are encouraged to attend a weekly seminar, which researchers from around the country and beyond are invited to visit the university and present their data. These seminars allow a broader understanding of the research being undertaken in lab groups around the world. We are also encouraged to attend the local Southampton Neuroscience Group (SoNG) seminar series, which offers the opportunity to learn about neurological pathologies and new discoveries, which are otherwise untouched or only superficially mentioned in lecture materials.The seminars provide a real insight into the complexities of designing controlled experiments and producing robust assays.
The novel and prominent aspect of our MNeuroSci course is the 'Advanced Neuroscience' module that was introduced this year. Through ten workshops with experts in a particular aspect of neurologically-based biochemistry, pathophysiology or pharmacology we are able to discuss, in depth, what is for many, a life’s work. A recent workshop explored the use of solid state NMR to elucidate the structure of disease-causing protein aggregates in the brain. The workshop brought to light the idea that distinct Amyloid protein structures could drive differences in pathological progression between patients suffering from Alzheimer’s. This discovery could facilitate personalised treatment plans dependent on a patient’s specific Amyloid fibril structure.
I believe that the final year of the MNeuroSci course prepares you far more thoroughly for the world outside of university than the initial 3 years of the degree. It is a comfortable transition, in which teaching through lectures and seminars is coupled with the opportunity to conduct your own scientific study. Fortnightly workshops enable real intimate contact time with lecturers, inspiring further research. Your daily workload is entirely up to you: it is your decision regarding how many seminars you attend, or how much writing or lab work you get done on any given day. As such, I am confident that the MNeuroSci course will unlock doors to many different research environments, and hence enable a more fluid conversion towards undertaking a PhD.
By Anna Bryans
On Saturday 6th February the Edinburgh University Neurological Society (EUNS) hosted their 4th Neuroscience to Neurology National Conference. The EUNS conference aims to bring together experts and students in the specialties of neuroscience, neurology and psychology.
The event proved to be our largest to date, attracting over 140 students from across the UK. The conference provided students the opportunity to present their research and we enjoyed a very high standard of poster presentations and oral presentations which covered a range of disciplines. In between student presentations, leading experts in the fields of regenerative neurology, psychiatry and the UK Biobank delivered engaging and thought-provoking lectures which highlighted the significance of both historical and existing advances in research. The afternoon workshops addressed diverse topics within neuroscience and neurology. They ranged from neurotrauma and nerve conduction studies to careers advice and the neuroscience of recovery. Prizes were awarded to the three top poster and oral presentations. Judges were also full of praise for the essay submissions to the EUNS essay competition which discussed “A historical figure in the field of Neuroscience, Neurology or Psychology”.
We are very grateful to all who participated and to the BNA for supporting our event. Overall, the conference emphasised how the integration of science, medicine and psychology can help us to understand how our brain functions in both health and disease.